Inhibitors of Platelet Integrin αIIbβ3
Continued examination of substituted 5H-[1,3,4]thiadiazolo[3,2-a]pyrimidin-5-ones as inhibitors of the platelet αIIbβ3 receptor resulted in the optimized agent ML165 (CID 44820665, NCGC00183896-01). This agent represents the most potent non-RGC mimetic inhibitor of the probe αIIbβ3 receptor, and due to its unique binding mechanism, offers a novel tool to study this receptor. Appropriate aqueous solubility and stability was found for this agent.
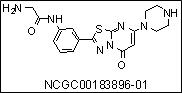
ML165
Key Investigators
National Center for Advancing Translational Sciences
Josh McCoy
Min Shen, Ph.D.
Wenwei Huang, Ph.D.
Craig J. Thomas, Ph.D.
Rockefeller University
Barry Coller, M.D.
Public Health Impact
This probe compound will be useful for studying selective inhibition of the αIIbβ3 receptor, which plays a pivotal role in hemostasis and thrombosis; deficiency of the receptor leads to Glanzmann thrombasthenia, and uncontrolled activation of the receptor produces thrombosis and blood vessel occlusion in animal models and humans.
Social Media Links