
September 2009
Insight
A Picture is Worth a Thousand Questions
An image, a protein and a driven scientist
By Allyson T. Collins, M.S., and Susan E. Johnson
NEI Science Writers

Pat Becerra, Ph.D., uses an automated perfusion chromatography system, which separates PEDF from other protein molecules.
Neither pay nor prestige drew Pat Becerra, Ph.D., to the National Eye Institute (NEI) in 1991. It was a single photograph that did the trick--a photograph of tumor cells from the eye responding to a protein that NEI researchers had recently discovered.
Tumor cells normally cluster together in a somewhat circular pattern, but these clusters of cells had extensions that looked like fingers reaching out to touch their neighbors. In fact, these tumor cells were behaving like nerve cells, which communicate through such extensions.
"When I saw this, I was so impressed," Becerra remembers about her first look at the image during a job interview at the NEI. "I wanted to know: How does the protein do this to the cells? What is this protein? How large is it? How is it folded? Where is it in the eye?"
At the time, she was studying HIV in a biochemistry laboratory at the National Cancer Institute, also part of the National Institutes of Health (NIH). She realized, however, that this protein was her chance to start from the ground up, and to ask basic questions about a little-known molecule.
"This was so compelling for me," she describes excitedly. "And what made it more interesting was the fact that the expertise I had in expressing proteins would be very useful for learning about this protein's function and potential therapeutic use."
Figuring out function
The protein was named PEDF, or pigment epithelium-derived factor, because it was found to be produced in a tissue beneath the retina known as the retinal pigment epithelium (RPE). The RPE nourishes the retina's light-sensitive photoreceptor cells to allow them to function properly.
Becerra's quest was to profile PEDF. Not long after beginning work at the NEI, she enlisted E. coli bacteria as her protein-making factories. She inserted the genetic code for PEDF into the bacteria, so they would produce a plentiful supply for her to study.
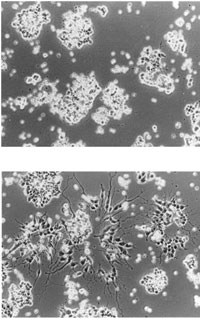
Top: Tumor cells from the retina, known as retinoblastoma cells.
Bottom: Retinoblastoma cells treated with PEDF protein change into nerve-like cells.
From: S. Patricia Becerra, Alessandra Sagasti, Patricia Spinella, and Vicente Notario. Pigment Epithelium-derived Factor Behaves Like a Noninhibitory Serpin. J. Biol. Chem., Oct 1995; 270: 25992-25999.
She found PEDF to be a large protein. Its structure resembled a family of molecules called serpins, which block the ability of enzymes to break down proteins. Serpins are involved in blood clotting, inflammation and the immune response, among other activities in the body.
But while PEDF looked like a serpin, it didn't act like one. "It didn't inhibit anything," Becerra says.
Meanwhile, in collaboration with other researchers at the NIH and around the country, Becerra's lab found something new that the protein could do--prolong the life of nerve cells growing in cultures.
"The cells would last longer--PEDF had a survival effect on the neurons," Becerra says. "So then other researchers started noticing, and asking, 'Can it do that for different types of neurons?'"
They found that the answer was yes; the protein protected all types of nerve cells. In the eye, PEDF delayed the death of light-sensitive photoreceptor cells in mice that had a form of retinal disease, and protected photoreceptors from light damage as well.
Another group of NIH researchers found that PEDF also prevents the growth and invasion of new blood vessels in the eye, which occurs in many blinding conditions, including a form of age-related macular degeneration.
"So then, with all of these interesting effects and activities, we were still asking the same question: how can it do all of this?" Becerra says.
The other half
Becerra's lab set off on a search for PEDF's modus operandi, keeping in mind that for the protein to work successfully, it probably needed a partner in crime.
"It has to bind to something," Becerra remembers thinking. "But where are the molecules that it interacts with? What are they? What are their functions?" She knew that once they found the location of PEDF in the eye, they would be close to finding the molecules with which it interacted.
"I want to ask questions," Becerra says. "Not to ask questions out of the blue, but so that somebody else can pick up my answers to solve problems."The researchers tagged PEDF and found that even though it frequented many locations, the protein primarily turned up in the RPE. Becerra's team also saw it move from the RPE to the photoreceptors in the retina--and, based on the images they obtained, they pinpointed locations where it could be binding to a receptor in the retina.
"That was a good association," Becerra says. "But we needed to find out more about the receptor."
Becerra's group was able to identify a new protein as the likely PEDF receptor. But they still needed to prove that PEDF actually interacted with this receptor. For this, they used a tool that allowed them to "watch" the molecules bind in real-time on a reflective gold chip, and to measure the bond's strength.
The analysis revealed that PEDF and the receptor candidate, which they called PEDF-R, had a very strong attraction--giving Becerra's team scientific proof that they had found the receptor.
A work in progress

Preeti Subramanian, Ph.D., (left) and Silvia Locatelli-Hoops, Ph.D., analyze proteins that have been separated in a gel and stained a blue color.
To determine the receptor's importance, Becerra has enlisted the collaboration of two postdoctoral researchers: Silvia Locatelli-Hoops, Ph.D., and Preeti Subramanian, Ph.D. Both have keyed in on the receptor to figure out if PEDF carries out its work through this molecule.
First, Locatelli-Hoops identified the precise binding location on the surface of the receptor--the specific point on a loop of amino acids.
"What I did was to dissect this loop into little pieces and screen all of them for binding to PEDF," she explains. "Only a small, central region of the loop binds." Subramanian then blocked this binding site, and discovered that PEDF's activity was weakened.
Though Becerra's group must do more experiments to prove that the receptor is critical for the actions of PEDF, these two key findings could give them and other researchers a larger picture of PEDF's role in the eye.
Questions to be asked
Nearly two decades after she interviewed at the NEI, Becerra now queries scientists who come to her own lab looking for a position. As she reflects on her research, she remembers a statement that she heard from a recent interviewee: "There are those who ask questions, and there are those who want to solve problems."
"I want to ask questions," she says. "Not to ask questions out of the blue, but so that somebody else can pick up my answers to solve problems."
Outside scientists are now maintaining Becerra's research momentum. One group is using the PEDF gene she discovered for phase I safety trials of gene therapy in the eye. These and other scientists hope to eventually capitalize on PEDF's protection of nerve cells and its anti-inflammatory properties to develop treatments for eye conditions such as macular degeneration and diabetic retinopathy.
"But all of that is outside of here," Becerra says, gesturing to the basic research laboratory surrounding her.
Inside her lab, with the Petri dishes, whirring machines, and chemical-filled vials, she still has plenty of questions to ask before she finishes the profile of the protein that drew her to the NEI.

