Medical Devices
Provision for Alternate Measure of the Computed Tomography Dose Index (CTDI) to Assure Compliance with the Dose Information Requirements of the Federal Performance Standard for Computed Tomography
Document issued on October 20, 2006
For questions regarding this document Stanley Stern at 301-796-5848 or stanley.stern@fda.hhs.gov.
 | U.S. Department of Health and Human Services |
Preface
Public Comment
Written comments and suggestions may be submitted at any time for Agency consideration to the Division of Dockets Management, Food and Drug Administration, 5630 Fishers Lane, Room 1061, (HFA-305), Rockville, MD, 20852.
When submitting comments, please refer to the exact title of this guidance document. Comments may not be acted upon by the Agency until the document is next revised or updated.
Additional Copies
Additional copies are available from the Internet. You may also send an e-mail request to dsmica@fda.hhs.gov to receive an electronic copy of the guidance or send a fax request to 301-847-8149 to receive a hard copy. Please use the document number (1609) to identify the guidance you are requesting.
Guidance for Industry, FDA Staff, and Third Parties
Provision for Alternate Measure of the Computed Tomography Dose Index (CTDI) to Assure Compliance with the Dose Information Requirements of the Federal Performance Standard for Computed Tomography
This guidance represents the Food and Drug Administration's (FDA's) current thinking on this topic. It does not create or confer any rights for or on any person and does not operate to bind FDA or the public. You can use an alternative approach if the approach satisfies the requirements of the applicable statutes and regulations. If you want to discuss an alternative approach, contact the FDA staff responsible for implementing this guidance. If you cannot identify the appropriate FDA staff, call the appropriate number listed on the title page of this guidance.
Introduction
This document provides guidance to manufacturers and assemblers of x-ray computed tomography (CT) equipment and to FDA staff. Also, it serves as information for:
- national and international organizations issuing safety and quality-assurance standards for CT equipment,
- professional organizations concerned with radiation protection,
- radiation safety personnel in the medical physics and health physics communities,
- staff at clinical facilities reviewing the radiation dose-related specifications of CT equipment, and
- physicians and x-ray technologists using CT equipment.
FDA is issuing this guidance to inform CT equipment manufacturers that it intends to exercise enforcement discretion, under certain circumstances, with respect to a specific provision of the U.S. Federal performance standard for computed tomography equipment (see the Code of Federal Regulations (CFR) at 21 CFR 1020.33). Specifically, FDA does not intend to object to the use of an alternate measure of the computed tomography dose index (CTDI). As discussed later in this document, CT equipment manufacturers who choose this alternative may substitute measured values of CTDI 100 for the required values of CTDI as defined in 21 CFR 1020.33(b)(1). No other provisions of the U.S. Federal performance standard are addressed in this guidance.
FDA's guidance documents, including this guidance, do not establish legally enforceable responsibilities. Instead, guidances describe the Agency's current thinking on a topic and should be viewed only as recommendations, unless specific regulatory or statutory requirements are cited. The use of the word should in Agency guidances means that something is suggested or recommended, but not required.
The Least Burdensome Approach
We believe we should consider the least burdensome approach in all areas of medical device regulation. This guidance reflects our careful review of the relevant scientific and legal requirements and what we believe is the least burdensome way for you to comply with those requirements. However, if you believe that an alternative approach would be less burdensome, please contact us so we can consider your point of view. You may send your written comments to the contact person listed in the preface to this guidance or to the CDRH Ombudsman. Comprehensive information on CDRH's Ombudsman, including ways to contact him, can be found on the Internet.
Background
New Aspects of CT Operation
Since the introduction of the concept of CTDI (Shope et al. 1981) and especially after FDA issued its formal regulatory definition in the Federal Register of August 31, 1984, advances in CT technology, practice, and radiation dosimetry have outpaced the accuracy of this quantity as an indicator of actual radiation dose and have weakened its relevance to clinical dose in patients (Dixon 2003). This situation is exacerbated by a widely held misperception of CTDI as an accurate measure of CT dose in individual patients and by its misappropriation for purposes of quality assurance and dose optimization in clinical practice (Brenner 2005). In fact, the quantity CTDI is an index which provides only an indication of the magnitude of doses that would be delivered to patients and of the changes in doses as a function of CT scanner model and conditions of operation. CTDI approximates the average central dose value associated with a spatially complex dose distribution in a reference acrylic dosimetry phantom for one particular set of exam techniques, i.e., those employing multiple, contiguous fan-beam scans in axial-scanning mode with fixed values of the x-ray tube current. Over the years, however, helical scanning, simultaneous acquisition of multiple tomographic sections, automatic exposure control, and cone-beam irradiation and detection geometry all developed as important new aspects of CT operation well beyond the scope of the axial-scanning mode appropriate for evaluation of CTDI.
Range of Mathematical Integration
In particular, as spatial resolution improved to accommodate imaging of tomographic sections significantly narrower than the 10-mm thick slices typical of CT systems and practice in the 1980s, the relatively short range of mathematical integration of CTDI in 21 CFR 1020.33(b)(1) limited the ability of CTDI to adequately account for contributions to dose from radiation scattered beyond that finite integration range.
Standardization
One response to these limitations was the adaptation by the medical physics community of the quantity CTDI 100 (and related variants), evaluated with a fixed-length (100 mm) “pencil” ionization chamber, as a more practicably measurable dose index (Leitz et al. 1995) and relatively more realistic indication of dose than the CFR-defined CTDI (21 CFR 1020.33(b)(1)) (Jessen et al. 1999). For measurements in the center of dosimetry phantoms, values of CTDI 100 are larger than those of CTDI by factors ranging from 2.6 to 1.0 for slice thicknesses ranging from 2 mm to 10 mm, respectively (Jessen et al. 1999). The adaptation of CTDI 100 was eventually standardized by the International Electrotechnical Commission (IEC 2002) and has been adopted by CT manufacturers and regulatory authorities internationally.
Advantages of CTDI 100
Although CTDI 100 in itself addresses few of the shortcomings of CTDI as a representation of dose to an actual patient, CTDI 100 has been a practical step forward from CTDI as defined in the CFR. When applied as intended to benchmark characteristic doses of different CT models operating in a conventional axial-scanning mode, either CTDI or CTDI 100 serves the same purpose. Either quantity in itself continues to be a useful, although narrow, means for comparison of relative dose efficiency scanner-to-scanner. However, CTDI 100 is much more broadly measured and applied than CTDI. More importantly, CTDI 100 also serves as the basis parameter in the evaluation of derivative indices used to refine the characterization of dose in CT. These latter indices are the weighted computed tomography dose index (CTDI w), the volume computed tomography dose index (CTDI vol), and the dose-length product (DLP) (European Commission 2004). Scanners complying with the current international safety standard for CT equipment display values of CTDI vol on their control panels (IEC 2002). Furthermore, CTDI vol and DLP are likely to be included in a CT standardized dose reporting module of the Digital Imaging and Communications in Medicine (DICOM) standard in the near future.
Recommendations for Harmonization
The National Conference on CT Dose Reduction, conducted in 2002 by the National Council on Radiation Protection and Measurements (NCRP), recommended standardization of CT dose terminology following input from a variety of national and international organizations concerned with standardization and radiation protection, including the National Electrical Manufacturers Association (NEMA) and the IEC (Linton and Mettler 2003). The recommendation to harmonize the U.S. Federal performance standard with standards of the IEC was echoed in meetings of the NEMA CT group with FDA staff in July 2003 and April 2004 as well as in follow-up correspondence.
Benefits
The substitution of CTDI 100 for CTDI will save manufacturers time and expense, with no reduction in safety, efficacy, or quality assurance of the equipment, because only one set of measurement values need be taken and provided to users to assure compliance with U.S. and international standards.
Use of CTDI 100
FDA intends to exercise enforcement discretion when CT equipment manufacturers substitute measured values of CTDI 100 for the required values of CTDI to meet the dose information requirements of the U.S. Federal performance standard at 21 CFR 1020.33(c)(2), if:
- the manufacturer’s substituted values meet the definition of CTDI 100 described below
AND
- the manufacturer clearly identifies the substituted values as CTDI 100 values rather than CTDI values.
Definition
Computed tomography dose index 100 (CTDI 100) means the integral of the dose profile along a line perpendicular to the tomographic plane divided by the product of the nominal tomographic section thickness and the number of tomograms produced in a single axial scan; that is:
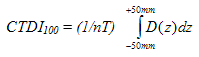
where:
z = position along a line perpendicular to the tomographic plane.
D(z) = dose in air at position z of the dosimetry phantom.
T = nominal tomographic section thickness.
n = number of tomograms produced in a single scan.
This definition includes the following aspects:
(1) It is assumed that the dose profile is centered on z = 0.
(2) Although D(z) is to be measured in a dosimetry phantom defined in 21 CFR 1020.33(b)(6) as made of polymethyl methacrylate (PMMA), dose is to be reported in terms of absorbed dose in air, not in terms of absorbed dose in PMMA. In this definition of CTDI 100, air serves as a reference medium while the PMMA dosimetry phantom serves as the actual material matrix within which measurements are made. (Note that this method of evaluation of CTDI 100 is contrary to that of CTDI, where D(z) is evaluated and reported as dose in PMMA.)
(3) For a multiple tomogram system, the scan increment between adjacent scans is assumed to be nT. When the scan increment between adjacent scans does not equal nT, an adjustment is to be made and explained in the user information. For example, for a CT scanner operating in a mode of overlapping tomographic sections such as that associated with a “flying focal spot,” it is assumed that the value of the product nT will be adjusted to account for the overlap.
CT-related terms used but not explicitly defined or explained in the preceding definition carry the same meanings as corresponding terms defined in 21 CFR 1020.33(b).
References
Brenner DJ. “Is it time to retire the CTDI for CT quality assurance and dose optimization?” Letter to the Editor, Medical Physics Vol. 32, No. 10, pp. 3225-3226, October 2005.
Code of Federal Regulations (21 CFR 1020.33, “Computed tomography (CT) equipment” April 1, 2006).
Dixon RL. “A new look at CT dose measurement: Beyond CTDI,” Medical Physics Vol. 30, No. 6, pp. 1272-1280, June 2003.
European Commission. 2004 CT Quality Criteria.
Federal Register . “Diagnostic X-Ray Systems and Their Major Components; Amendments to Performance Standard,” August 31, 1984 (64 FR 34698).
International Electrotechnical Commission. International Standard IEC 60601-2-44 Edition 2.1, Medical electrical equipment – Part 2-44: Particular requirements for the safety of X-ray equipment for computed tomography, November 2002.
Jessen KA, Shrimpton PC, Geleijns J, Panzer W, and Tosi G. “Dosimetry for optimisation of patient protection in computed tomography,” Applied Radiation and Isotopes Vol. 50, No. 1, pp. 165-172, January 1999.
Leitz W, Axelsson B, Szendrö G, “Computed tomography dose assessment: A practical approach,” Radiation Protection Dosimetry Vol. 57, pp. 377-380, 1995.
Linton OW and Mettler, Jr., FA. “Opinion. National Conference on Dose Reduction in CT, with an Emphasis on Pediatric Patients,” American Journal of Roentgenology Vol. 181, pp. 321-329, August 2003.
Shope TB, Gagne RM, and Johnson GC. “A method for describing the doses delivered by transmission x-ray computed tomography,” Medical Physics Vol. 8, No. 4, pp. 488-495, July/August 1981.