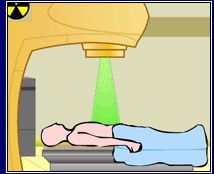
scene from animation: patient undergoing radiation treatment for cancerous tumor
Over 10 million people in the U.S. — about one in 30 — are cancer survivors. This growing population reflects advances in cancer detection and treatment. But with the greater number of survivors comes an increasing number of people living long enough to experience more than one type of cancer in their lifetime. Overall, cancer survivors have a 14 percent higher risk of developing a new primary malignancy compared with the general population, according to a new NCI monograph entitled, New Malignancies among Cancer Survivors: SEER Cancer Registries, 1973-2000. The risk factors involved in the second cancer may be the same as those that led to the original tumor, such as smoking, excessive alcohol use, diet and nutrition, and genetic predisposition. For some people, however, the radiotherapy or chemotherapy received to treat the first cancer may be a contributing factor for their developing a completely new primary cancer.
Radiation therapy, or radiotherapy, has been used routinely to treat certain types of cancer since the 1920s. The most common forms of radiotherapy are external beam therapy, in which radiation is administered from an external source, and brachytherapy, in which the radiation source is placed within body cavities or tissues. Radiotherapy can completely destroy a tumor, shrink it enough to alleviate symptoms, or reduce the size of the tumor so it can be removed by surgery. Radiation is used to treat many cancers such as oral cavity, esophagus, respiratory tract, female genital tract, testis and brain cancer, as well as lymphoma. Radiotherapy may be used alone or in combination with other cancer treatments, such as chemotherapy, hormonal therapy, or surgery.
Despite their efficacy, cancer therapies can act as carcinogens, or cancer-causing agents. Their use may contribute to cancer survivors’ increased risk for developing a second cancer, even years after their original treatment. It is important to emphasize, however, that in most cases, the benefits of treatment greatly outweigh the risk of treatment-related second cancer, and it is only because of the successes in treating so many cancers that the long-term adverse effects of therapy are becoming more apparent.
Radiation-related solid cancers
Epidemiologic research has demonstrated that ionizing radiation in the form of radiotherapy accounts for the elevated risks of certain second cancers. An individual’s risk of radiation-related, or radiogenic, second cancer depends on several critical, often inter-dependent factors: the type and dose of radiation received; the amount of the body treated (treatment field); the part of the body irradiated and its inherent tissue sensitivity; and the age at irradiation.
Individual organs and organ systems can vary in sensitivity to the effects of radiation exposure. Some organs, like the prostate, are relatively radiation-resistant, while others are considered radiation-sensitive. Several epidemiologic studies of radiation-exposed populations, including Japanese atomic bomb survivors and patients receiving radiation therapy, have shown that the breast, thyroid, and lung are particularly sensitive to the carcinogenic effects of radiation. Radiation-related solid cancers are often not detectable until at least 5, 10, or even 20 years after initial exposure to radiation. This long latency period (time between exposure and cancer diagnosis) points to the need for long-term surveillance of cancer survivors who have received substantial radiation exposures. Other factors, such as genetic susceptibility, may also influence the time to appearance of radiation-induced solid cancers.
Age at which radiation exposure occurs is also a strong factor in radiation-induced cancer. Radiation sensitivity is especially pronounced in the tissues of children. “A child is a growing organism, and during times of rapid growth there is thought to be more cell turnover, and therefore more chances that genetic alterations could be induced over time,” explained Margaret A. Tucker, M.D., chief of the Genetic Epidemiology Branch in NCI’s Division of Cancer Epidemiology and Genetics (DCEG). Because of their age at treatment, childhood cancer survivors have an especially increased risk for other cancers later in life. “People who survived cancer as children live for a much longer time than do older adult survivors, so there is more time for radiation-related complications to manifest themselves,” continued Tucker. Radiation therapy used to treat childhood cancers has been associated with increased risks of subsequent cancers of the thyroid, breast, bone, and brain, when these regions are exposed to substantial radiation doses. The chance a childhood cancer survivor receiving cranial irradiation will develop a brain tumor later in life is also related to age at radiotherapy, with those treated before the age of five years having the highest risks.
Adolescents and young adults are also particularly prone to the late effects of radiation. In a large survey of women treated for Hodgkin lymphoma, breast cancer was the most frequently diagnosed solid tumor; risks were markedly higher in women who were treated at or before the age of 30. These risks are typically attributed to high-dose, large-field irradiation to the chest. “In a subsequent, large international investigation in which the roles of both radiotherapy and chemotherapy were studied in detail, we found that the risk of breast cancer significantly increased with increasing radiation dose to the area of the breast where the cancer was later diagnosed,” said Lois B. Travis, M.D., a senior investigator in the Radiation Epidemiology Branch in DCEG. “Conversely, the risk of breast cancer significantly decreased as the number of cycles of chemotherapy increased,” stated Travis. ”The reductions in breast cancer risk roughly paralleled the percentage of women who became menopausal after treatment.” This protective effect of chemotherapy likely reflects the diminished hormonal production resulting from treatment-related ovarian damage, and it further illustrates the complexity of determining late treatment effects.
In adults, increased risks of radiation-related cancers have been found for specific cancer types. For example, excess risks have been seen for cancers of the lung and esophagus, as well as sarcomas, after initial radiotherapy for breast cancer, and high dose radiotherapy for cervical cancer has been linked to elevated risks of subsequent cancers of the stomach, rectum, bone, urinary bladder and other sites.
Some patients with rare inherited genetic mutations may be predisposed to developing second cancers after radiotherapy. Researchers have found that survivors of hereditary retinoblastoma, a rare childhood cancer of the eye caused by inherited mutations of the Rb-1 tumor suppressor gene, have an elevated risk of developing bone and soft tissue sarcomas, especially after they received radiotherapy. Retinoblastoma survivors without an inherited mutation are not at increased risk for developing second cancers.
Radiation-related leukemias
Although more commonly associated with chemotherapy, some types of leukemia, such as acute myelogenous leukemia, chronic myelogenous leukemia, and acute lymphocytic leukemia, may arise after cancer treatment with radiation. Radiogenic leukemia may be detected soon after exposure, as early as two years after treatment, although the increased risk typically peaks between five and nine years after irradiation and slowly declines thereafter. Several factors are thought to affect risk, including radiation dose to the bone marrow, and the percentage of bone marrow exposed. Studies of cancer patients have found that the risk of leukemia may level off or even decrease after high doses of radiation exposure to the bone marrow, possibly due to the destruction of bone marrow cells, or a “cell killing” effect. In contrast, studies of testicular cancer survivors have reported sizable leukemia risks associated with high-dose radiotherapy to the chest, abdominal and pelvic fields, which caused large volumes of bone marrow to be exposed to radiation.
Hope for the future
Radiation-related second cancers provide a unique opportunity for studying the development of cancer following a known dose of a specific carcinogen. Long-term studies of cancer survivors have quantified the relationship between the amount of radiation received in treatment of the initial cancer and the risk of developing a new one. Studies of children and young adults suggest that for many solid tumors, the incidence of radiation-related cancer tends to increase with increasing radiation dose. This evidence has encouraged researchers to search for ways to reduce radiation doses, while maintaining treatment efficacy, in order to decrease the long-term risks of solid tumors. As advances are made in radiation therapy techniques, physicians are becoming better able to tailor treatments strategies to minimize the radiation dose delivered and carefully direct radiation therapy to pinpoint a tumor while avoiding healthy tissue.
In addition, the more scientists learn about carcinogenesis, the better able they will be to develop biomarkers which will help identify patients who would most benefit from specific treatments, and common genetic variants that may modify the late effects of radiotherapy and other treatments. In the future, these technologies may allow physicians to personalize treatment plans to reduce the risks of second cancers associated with radiotherapy. “Prospective identification of patients genetically susceptible to the late complications of cancer treatment could result in opportunities to individualize therapy to not only maximize therapeutic benefit, but to minimize all types of serious late toxicity, including second cancers,” notes Travis.
Animation/Video
| This animation requires the Flash plug-in. If you do not have the plug-in, please click here to install. | |
Text Transcript
Second Cancers
Some anti-cancer therapies can damage DNA in healthy cells and lead to the development of second cancers.
Tumor Formation
Cancer cells grow and divide rapidly, without control. In healthy cells, normal control mechanisms are in place to regulate these processes.
Radiation Therapy
Sometimes cancer is treated by directing beams of radiation at the site of the tumor.
Chromosomes
In cells, DNA is wound up tightly and packed into chromosomes, located in the nucleus.
Radiation Damage
Radiation can damage DNA by inverting genes or translocating whole chunks of genetic material from one chromosome to another.
Therapy-induced Second Cancers
If DNA damage caused by radiation or chemotherapy cannot be repaired, the cell might destroy itself. If it is a cancer cell, tumor growth is inhibited and the disease is treated. Sometimes healthy cells incur damage and a new cancer develops months or years after treatment for the first tumor. Second cancers can form in the same organ or in an entirely different part of the body.
Photos/Stills
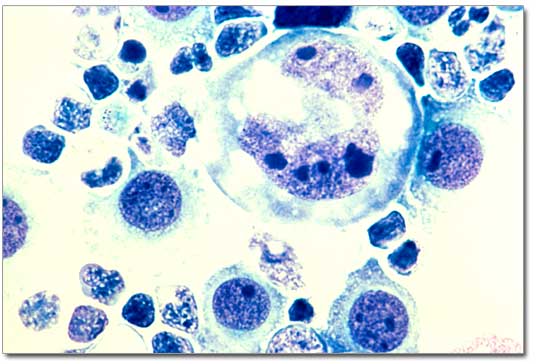
1. Lymphoma cells under the microsope
2. Radiation treatment for retinoblastoma in children

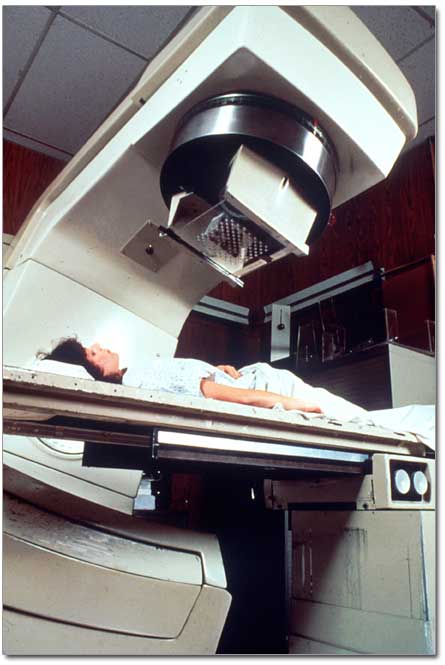
3. Radiation therapy
 NCI NewsCenter
NCI NewsCenter NCI Budget Data
NCI Budget Data Visuals Online
Visuals Online NCI Fact Sheets
NCI Fact Sheets Understanding Cancer Series
Understanding Cancer Series