Clinical Trials Management Systems Knowledge Center
Dear CTMS Knowledge Center community, As of May 11, 2012 the CTMS Knowledge Center, as with all caBIG® Knowledge Centers, will no longer be operated by dedicated Knowledge Center staff. The online resources associated with the Knowledge Center, such as forums and wikis, will remain operational; however, new content on them from this date will be 100% community-provided, rather than provided by individuals and groups that are funded by NCI to do so. The Knowledge Center sites are open and free for all to use, and we encourage your continued active participation, so that users may continue to ask, and obtain answers to, pertinent questions relating to these informatics capabilities. Furthermore, please note that the former Knowledge Center personnel are free to continue to post to the Knowledge Center online resources as community members; they are simply no longer paid to do so. In addition to the peer support made possible by the online resources of the Knowledge Centers, triaged direct support remains available from NCI Application Support which, as always, can be contacted by e-mail at ncicb@pop.nci.nih.gov or by telephone at 301-451-4384 (toll free: 888-478-4423). Support is available Monday to Friday, 9 a.m. to 5 p.m. US Eastern Time, excluding government holidays. NCI’s Center for Biomedical Informatics and Information Technology (CBIIT) expects to continue the provision of informatics support currently provided by the Knowledge Centers, in a manner consistent with such feedback as we obtain from the NCAB ad hoc Informatics Oversight Committee, soon after the launch of the new National Cancer Informatics Program (NCIP). NCIP is not a renaming of the caBIG® program, but rather an opportunity to reevaluate and reshape how NCI supports the informatics needs of the cancer community. If you would like to voice concerns, questions or suggestions beyond immediate requests for support, please contact Anthony Kerlavage (anthony.kerlavage@nih.gov). |
About the CTMS Knowledge Center
The Clinical Trials Management Systems (CTMS) Knowledge Center provides a centralized, authoritative repository of knowledge, information, and web-based support to facilitate the deployment and ongoing development of tools, standards, and infrastructure in the CTMS domain. The Knowledge Center provides a platform for fostering open source development of CTMS tools.
What's New?
Important things to know about the current availability of CTMS tools.
Tools and Products
caBIG® Clinical Trials Suite
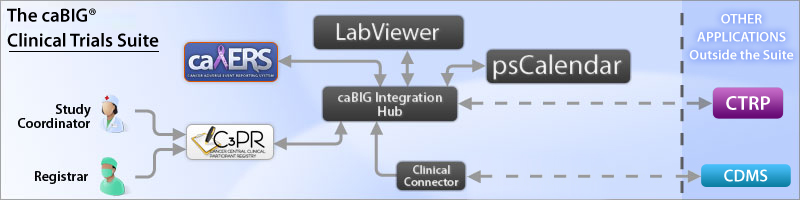
The caBIG® Clinical Trials Suite (The Suite) is an enterprise clinical trials system that has been developed (and continues to be enhanced) primarily for use in trial sites. The following diagram shows the tools in The Suite and the relationships among them.
Visit the caBIG® Clinical Trials Suite demo site where you will find login information.
Tools in the caBIG® Clinical Trials Suite
The Study Coordinator and Registrar provide participant registration information to the caBIG® Central Clinical Participant Registry (C3PR). C3PR interfaces with the other applications, and they interface with each other, and with other applications, via the caBIG® Integration Hub (formerly caXchange).
[caBIG® Patient Study Calendar (PSC)] provides the means to manage schedules of participants centrally. caBIG® Adverse Event Reporting System (caAERS) provides the means to collect and manage adverse event data.
caBIG® Lab Viewer (formerly CTODS Lab Viewer) is used to view laboratory data in transit between clinical laboratory and clinical data management systems. caBIG® Clinical Connector (formerly C3D Connector) provides a semantically integrated service layer via caGrid that allows users of a Clinical Data Management System (CDMS) to expose functions within the CDMS.
The following are provided as standalone tools, as well as tools that are part of the Suite.
- caBIG® Adverse Events Reporting System (caAERS) - Demo site; Username: SYSTEM_ADMIN, Password: system_admin
- caBIG® Central Clinical Participant Registry (C3PR) - Demo site; Username: c3pr_admin1, Password: c3pr_registrar
- [Patient Study Calendar] (PSC) - Demo site; Username: superuser, Password: superuser
Additional CTMS Tools and Products
Demonstration Sites
Access Information |
caBIG® Clinical Trials Suite |
caAERS |
C3PR |
PSC |
Lab Viewer |
OpenClinica |
|---|---|---|---|---|---|---|
Demo Site URL |
||||||
User Name |
SYSTEM_ADMIN |
c3pr_admin |
superuser |
lbdtusr001 |
cdmsadminoc |
|
Password |
— |
system_admin |
c3pr_admin |
superuser |
!Q@W3e4r!! |
!Q@W3e4r!! |
CTMS Projects
- CTMS Workspace
- CTMS Workspace and Projects Wiki
- Business Architecture Model (BAM)
- Interoperability Scenarios
- BRIDG Model