About FDA
FDA Basics: Vaccines, Blood, and Biologics
FDA Basics
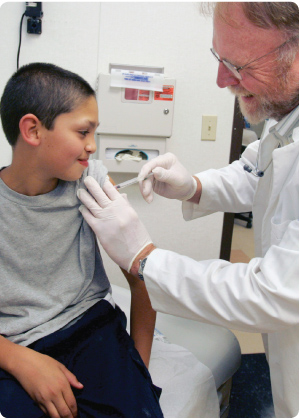 | Vaccines, blood, and biologics are regulated by FDA's Center for Biologics Evaluation and Research (CBER). CBER protects and advances the public health by ensuring that these products are safe and effective and available to those who need them. CBER also provides the public with information to promote the safe and appropriate use of these products. |
Do You Know…
How FDA Assesses the Safety of Vaccines?
Vaccines undergo rigorous and extensive testing to determine their safety and effectiveness. Highly trained scientists and medical personnel at FDA carefully review all of the information in a marketing application before a vaccine can be approved for use by the public. Following approval, FDA also carefully monitors the quality of vaccines. More…
FDA Basics Videos
Norman Baylor, former director of the Office of Vaccines Research and Review in the Center for Biologics and Research, talks about H1N1, vaccines, and protecting human life. Watch FDA Basics Videos...
|
Information for Blood Donors
If you would like to ask a specific question, please visit our "Contact Us" page for more information about how to contact FDA.
Please note that any information you submit may become public or subject to release under the Freedom of Information Act (FOIA). For more information, read about our privacy policies and the FOIA.