Plant fatty acids are used in a vast range of products, from polymers to plastics and soaps to industrial feed stocks -- making up an estimated $150 billion market annually. A new discovery of inserting double bonds in the fatty acids could show the way to the designer production of plant fatty acids, and, in turn, to new industrial applications and new products. Read more.
A new catalyst can store hydrogen fuel in watery solution under mild temperatures and pressures with no toxic byproducts, and at a faster rate than any previous catalyst.
A highly efficient catalyst to convert renewable crops into the product propylene glycol was discovered by scientists at the Pacific Northwest National Laboratory and commercialized by the Archer Daniels Midland Company.
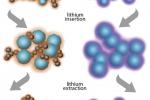
In the U.S., businesses tend to invest in research that will pay off in the short term. National laboratories are filling a gap by conducting the essential research that will change the game 10 to 20 years down the road. Learn more about how years of conducting advanced research in both the private and public sectors led to battery technology that made electric cars possible.
Teams at two of the Energy Department's laboratories are making headway on two projects that will enable building a new lithium battery that charges faster, lasts longer, runs more safely, and might also arrive on the market in the not-too-distant future. Learn more.