Cancer in Populations
Does smoking cause lung cancer? Do high-fat diets increase the risk of breast cancer? Is arsenic in water linked to cancer? These are the kinds of questions that epidemiologists try to answer. In this month’s Benchmarks, we talked to one of NCI’s leading epidemiologists, Dr. Robert N. Hoover, about epidemiology in general, and about the research in NCI’s Division of Cancer Epidemiology and Genetics (DCEG). DCEG is the primary focus within NCI for population-based research on environmental and genetic determinants of cancer.
Robert N. Hoover, M.D., Sc.D., is the director of DCEG’s Epidemiology and Biostatistics Program. Dr. Hoover serves on many national and international committees concerned with various aspects of epidemiology and preventive medicine. He was awarded the Public Health Service Commendation Medal in 1976, the Meritorious Service Medal in 1984, and the Distinguished Service Medal in 1990. In 1996, he received the Gorgas Medal for distinguished work in preventive medicine from the Association of Military Surgeons of the United States. His most recent awards include the Distinguished Service Award, DES Action, 1999; Public Health Service Physician Researcher of the Year, 2001; The John Snow Award from the American Public Health Association, 2001; The Distinguished Achievement Award from the American Society of Preventive Oncology, 2002; and The Harvard School of Public Health Alumni Award of Merit, 2002.
What are the current research priorities of your division?
Dr. Hoover: Historically, we have built a program which is both very broad and very deep. Because we are the National Cancer Institute, we don’t have the luxury of focusing on only one disease or one exposure. We are expected to have a broad program that encompasses all the major areas that are thought to be interesting and fruitful in the epidemiology of cancer. So we have large programs in genetics, occupation, radiation, nutrition, hormones, viruses, biostatistics—our programs cover the waterfront.
At the same time, we try to be at the cutting edge of research in each of these programs—to have something going on that will move the field forward. We try to take advantage of the opportunities of the time. The current opportunities are in interdisciplinary studies, biochemical or molecular epidemiology. Advances have been made in the laboratory during the last 10 or 15 years and there are new ones almost every day that are very useful in our kind of work. These allow us to identify people who are exposed to specific agents and people who may be susceptible to diseases, or to get new insights into mechanisms involved in carcinogenesis. There’s a lot of enthusiasm and a lot of opportunity for combining robust epidemiology research and advanced laboratory tools to take advantage of both disciplines. These new tools influence each of our programs.
What’s an example?
Dr. Hoover: There’s a whole lot of interest in environment-gene interactions, identifying genetically-susceptible states, and using genetic alterations to discover previously unrecognized or unappreciated environmental exposures. We’re involved in a major attempt to do this right now with breast cancer and prostate cancer in something called the Cohort Consortium. This program is an attempt to put together several of the large follow-up studies in the world—a couple at NCI, some from Harvard, some from Europe, some from Southern California, and the American Cancer Society. All of these cohorts have information on exposures and the development of disease, as well as blood specimens. In total, there are 7000 cases of breast and 7000 cases of prostate available for study. We’re going to focus on an area which has been known for a long time to influence these diseases—hormones. We’re going to use the opportunity to look at genes and hormone exposures together to try to learn something useful in terms of prevention. We want to find out how these hormonal exposures might work, what the real levels of the hormones are, what dictates the levels—including exogeneous sources like drugs, as well as endogenous sources. Also, we want to know how they interact with each other and with growth factors, and basically try to understand the genetic and environmental determinants of these two major cancers. This is indicative of the kinds of things we are doing throughout the program.
Another example is a bladder cancer study in New England. This is an interdisciplinary study looking for clues to the high rates of bladder cancer in New England. We noticed many years ago in doing cancer maps that bladder cancer concentrates in major urban areas. This is what we expected given the relationship between tobacco use and occupational chemical exposures and risk of this malignancy. However, we felt that this wouldn’t explain the very high rates also seen in rural areas of New England. This has been a source of concern for some time, and we’ve been searching for a hypothesis that we can pursue.
Then some other work we did for bladder cancer and some work from Taiwan suggested that drinking water may be a candidate. It’s something that both sexes consume and widespread exposures, even if they were associated with relatively small increases in risk, could influence general population rates. These studies suggested that arsenic could cause bladder cancer. Arsenic can leach into the ground water from granite sources, which are abundant in New England. This has provided us with a hypothesis. We are working with Dartmouth University, the state of New Hampshire, the state of Vermont, the state of Maine, the U.S. Geological Survey and anyone that can help us understand water availability patterns. We are assaying arsenic in drinking water from wells and community water supplies. We’re looking at a battery of potential susceptibility genes, along with other risk factors that may have a role, such as occupational exposures and tobacco. So, it’s a pretty ambitious undertaking. Having the tools to measure exposure, as well as susceptibility, makes us excited that we can evaluate at least some of these hypotheses.
On your web site, you state that the research philosophy of your division is to serve as a “national program for population-based studies to identify environmental and genetic determinants of cancer.” How does being a national program affect the focus of your work?
Dr. Hoover: Looking historically around the world, epidemiology has always had a strong presence in governments, and particularly in national governments. The primary reason for that is that it’s concerned with epidemics, which is a concern of government. But, there is another reason as well. In terms of research, epidemiology is different from laboratory research—you can’t just do it anywhere. You need populations–large populations–that have undergone natural experiments—that have had unusual exposures, or have high or low cancer rates. A national perspective is helpful in responding to these problems and opportunities. We have access to national data resources, such as the National Center for Health Statistics, population-based tumor registries, the social security administration, medicare, and agencies that collect large amounts of data that can be used for epidemiological ends. We can move easily to where the action is. We can look, for example, at lung cancer in Glynn County, Georgia, or colon cancer in David City, Nebraska. We can and have gone to these and other places, found collaborators in local health officials and initiated studies to investigate high rates of diseases in those areas. It’s relatively easy for us to do that.
You can probably afford to take scientific risks?
Dr. Hoover: Yes. We are encouraged to do things that would be relatively risky for academics to do—perhaps a five-year study that has a high probability of coming up with nothing, but if it did come up with something, it would be extremely important for public health. Oftentimes we’re put in those roles. We have the stability to do that and don’t have to stake anyone’s entire scientific future on one particular study.
DCEG’s research program is international in scope. Where and why is DCEG carrying out epidemiology research around the globe?
Dr. Hoover: We are an international program for a couple of reasons. We are in the federal government and we get involved with bilateral and other nation-to-nation, government-to-government agreements. We were some of the first U.S. scientists in China after president Nixon’s opening up of that country, and our relationship with the Chinese National Cancer Institute was one of the first scientific bridges to that country. We were prominent in détente initiatives with the Soviet Union and Soviet bloc nations, and we currently have a role in the Middle East Cancer Consortium, and in forming the Ireland-Northern Ireland-NCI Cancer Consortium.
Because of our position, we have an opportunity to take advantage of major disease differences that occur throughout the world. We do work in an area of China, where 25 percent of the population develops esophageal cancer. We do work in Latin America, where the cervical cancer rates are the highest in the world. We also take advantage of international differences in availability of resources. For instance, we collaborate often with Scandinavian countries, which have record linkage resources, and we use these records as resources for testing epidemiology observations. Many countries have single-payer health care systems—i.e., the government—which makes it a lot easier to do health investigations because you’re dealing with only one health care delivery system.
Right now, we’re doing a very large study of benzene workers in China. We’re trying to investigate what levels of benzene are related to a variety of cancer risks. Many factories in China use benzene in a variety of activities, so there’s a range of exposures and there’s a great collaborative spirit to allow us to measure levels. We’ve also done a variety of studies of cervical cancer in Latin America. Our work there allowed us to identify the major role of human papillomaviruses (HPV), as well as the specific subtypes of HPV responsible for the disease. Because of an extremely productive research collaboration with investigators in the government of Costa Rica, we are launching a major vaccine trial with a candidate vaccine against HPV developed by NCI researchers.
Are there any fundamental differences in design between epidemiological studies focused on genetic factors and those concerned with environmental factors?
Dr. Hoover: For the most part, the methods in human populations are quite similar. There are some specific methods that are different. For example, family studies are used to identify high penetrance genes-the genes that are responsible for familial cancers. But, these kinds of studies are not very productive for studying environmental exposure because most families tend to have the same environmental exposures. Outside of that, trying to evaluate the impact of natural genetic variation in the population on cancer risk is very similar to looking at environmental exposures. It’s essentially another exposure–you are exposed to a particular version of a gene.
One difference, however, is a difference in scale. In environmental studies, we worry about confounding exposures. So, if you’re investigating coffee drinking, you have to control for the effects of tobacco because they usually correlate. But, there are usually a limited number of confounding factors that you know about or that you can assess. With the new genetic technology, there is a new possibility that if you’re interested in a particular gene, there may be thousands of others whose effects you may be called upon to control for. This does serve up a kind of challenge of scale that we haven’t seen before. In general, however, the principles are largely similar and transferable.
What are the priorities for future epidemiological studies?
Dr. Hoover: Epidemiology is an opportunistic science. It goes where the action is not only in terms of disease and exposure, but also where the tools are. Many epidemiologists are most anxious to use the new molecular tools to assess exposures better, as well as measuring susceptibility. For example, it would be wonderful to have a biological dosimeter for your exposure to benzene from gasoline fumes, or your lifetime level of consumption of fat in your diet. It’s difficult to get at these kinds of exposures by asking questions. We are hopeful that the emerging technology from measurement science will provide opportunities in this area. That is an area all of us hope will come to fruition.
You mean that by assaying blood or DNA samples you can estimate what someone’s exposures have been?
Dr. Hoover: Correct. For example, in the area of diet–dietary fat exposure: People have difficulty giving an accurate assessment of their diet last week, let alone their lifetime exposure. Getting an assessment of lifetime dietary fat exposure is extremely difficult to do with current tools. However, if there were some change in your metabolism or some other biologic alteration that would reflect your long-term exposures to specific nutrients, that would be extraordinarily useful. Right now, it’s mostly talk.
It’s part of one of the extraordinary opportunities in the bypass budget. We’re trying to encourage people who work in those areas of technologies. We need close collaborations between people working in basic science, chemical carcinogenesis, nutrition, and technology development in order to make progress.
What are some of the landmark epidemiological studies that have been published during your career?
Dr. Hoover: Most of what we know about human cancer has come first from studies in human populations–either clinical observations or epidemiology studies. It wasn’t until the late 1960s and early 1970s that the whole range of radiation-related solid cancers (other than leukemia, which was known earlier) began to be recognized, primarily from a whole series of outstanding work done with atomic bomb survivors. Then there was a series of discoveries linking infectious agents to cancer–Hepatitis B and liver cancer, HPV and cervical cancer, HTVL-1 with leukemia and lymphoma. In the occupational setting, in the late 1960s, there was a famous study showing increased lung cancer rates in steel workers working near coke ovens. This was followed by studies showing the leukemia risks of benzene exposure, and a number of studies indicting a variety of other occupational carcinogens, even up to the recent studies of dioxin. And in the area of drugs, one of the most important findings for public health was understanding the role of HRT and breast cancer risk. Another important study showed the connection between immunosuppressive drugs and non-Hodgkin’s lymphoma. Through the study of Li-Fraumeni families, Joe Fraumeni, Fred Li, and Steve Friend used the tools of the genetic revolution to give us insights into the role in humans of the tumor suppressor gene p53, which is relevant to a whole variety of cancers. In the area of genetics, other noteable advances included the discovery of BRCA1 & 2 and a whole series of cancer susceptibility genes.
How do the challenges of doing epidemiology research today compare to when you first arrived at NCI in 1972? Do the research tools available today make it easier to do research? What are some of the problems?
Dr. Hoover: There’s been a lot of advancement in measuring exposures and in ways of collecting history of exposure. Also, all of the current molecular tools and ways of measuring things in blood and other biologic specimens make it easier to do research today. There’s also been a whole series of statistical advancements both in our ability to sample populations and to analyze data. The tools of the trade have gotten a lot better and continue to improve.
On the other hand, it’s been more difficult to do some aspects of epidemiology because of changes in our culture. In the past, it was relatively easy to get 80 percent of the normal population to cooperate in studies as controls. It’s extremely difficult now. People are so assaulted with telemarketers and fund-raisers that researchers become painted with that brush when we try to call on the phone, send letters, get answers to questionnaires, get people to give blood and, generally, do things for medical research. People are less responsive. In addition, the burgeoning concern over privacy and confidentiality issues make it much more difficult to do these studies now than in the past.
Also, historically there has been a focus on risk factors that are associated with major increases in risk. For example, we know that tobacco gives you a 10-fold risk of lung cancer and HPV a several hundred-fold increased risk of cervical cancer. More recently, much work has begun to focus on a concern on the part of scientists that many risks may be the sum total of several minor risks–on the order of 10 percent or 20 percent, rather than 1000%. These are tough. It’s tough to measure the relevant exposure, to distinguish high vs. low exposures. And the result you get could be due to confounders, whose effects are much more difficult to exclude when dealing with relatively low levels of risk. There’s been a whole lot of interest and a whole lot of statistical work in small risks, but it still remains a major challenge for public health.
How do epidemiology results change public health? How cautious does the public need to be in interpreting preliminary results from epidemiological studies?
Dr. Hoover: For better or for worse, epidemiology results generally get out to the public quickly because they’re understandable. They don’t use arcane language; they deal with real people and real exposures that real people get. Therefore, they are instantly relevant and recognized. As happens in science in general and in medicine in particular, there is an increasing interest on the part of lay press to move information to the general public quickly and in great volume. It is no longer the case that you can publish something that you would like to see replicated two-three times before signaling any public health concern. That’s difficult to do these days because once you publish, it’s out. It’s of interest. The concern that many people have is that because of this there is lack of discrimination for what is a solid finding vs. not-so-solid finding, or what are ones that do and do not deserve action. This applies to all medical science. It applies to findings in petri dishes for new cancer treatments but, in fact, it’s more prevalent in epidemiology because it’s instantaneously recognized as a real people finding.
We’re not going to change the openness of society, or the interest of people in these issues or the aggressiveness of media. We’re hoping that education will out–the education of the media on reporting the finding, the education of scientists on how to report, the education of the public on how to interpret and deal with the avalanche of information. Certainly there are misrepresentations of the solidness of findings. The responsible parties are the scientist, the public, and the media. There are opportunities for misrepresentation at each of those levels.
I think that this is not all as bad as some people paint it. I think it’s a process of learning how to communicate the information better. And the public is learning. I have found in my career describing the relationship between menopausal hormones and breast cancer that when I have the opportunity to speak to people (either the general public or reporters on the phone), everyone is smarter than we give them credit for. They understand the level of evidence and what the choices are when you take some time to talk with them. They can handle uncertainty and the scientific method if it’s delivered to them in an understandable manner. I have a lot of faith and reason to believe that the public can deal with information if it is delivered in a reasonable way.
Dietary fat and breast cancer. Where are we now with that issue?
Dr. Hoover: We’ve been all over the map with that. There is still overpowering descriptive data that diet probably is involved in a substantial amount of breast cancer risk. However, very little is known about what specific aspect of the diet is involved. The sum total of the data currently doesn’t favor a major role for dietary fat, but certainly over-interpreted data could lead to that conclusion. The initial data were international rates: U.S. and Western Europe have high rates of breast cancer; Japan and China have low rates of breast cancer; therefore dietary fat is the cause. That kind of loose thinking led to a lack of distinction about levels of evidence.
In the late 1940s, there was concern about the cause of the major increase in lung cancer. One very prominent hypothesis was that the cause was asphalt roads. There certainly was a correlation. There were a lot of roads where the lung cancer rates were high. But correlations aren’t the same as causes. Tobacco was shown to be the cause.
Epidemiology has often been considered the field of research that can suggest causation, whereas clinical studies and laboratory experiments are really needed to prove causation. What do you think?
Dr. Hoover: That’s clearly false. It depends on your definition of proof. The weight of the evidence from human studies can achieve a level where people are willing to act on the fact that it’s likely to be a cause, and the result of those actions are the ultimate test of that causality. I have a very simple definition of cause myself. That is: If you remove the exposure, the disease goes away, and if you introduce the exposure, the disease appears.
Now, you can do that in a randomized trial, for example, giving drugs which are thought to be chemoprevention agents. Or you can say the weight of the observational evidence is sufficient, such as the evidence that tobacco is related to a large number of cancers: the weigh of the evidence is so high you don’t need a trial–you need to get rid of the exposure. Remarkably, as we got rid of the exposure, the rates for these cancers started plummeting. Does this mean that I understand the mechanism by which tobacco smoke produces cancer in humans? No, it doesn’t because I don’t. And actually, neither do my laboratory colleagues, although they have a myriad of hypotheses. But probably neither they nor I need to know that in order to accept causality.
It’s also possible that good, solid laboratory findings can suggest that an exposure might protect against cancer, and human observations might determine that that’s not the case. On the other hand, good solid human epidemiology can suggest that something is a carcinogenic exposure, and then interventions determine that that’s not the case.
Are there any examples where epidemiology suggested doing X and you did X and the rates didn’t go down?
Dr. Hoover: Yes, beta carotene [a vitamin A precursor] and the lung cancer prevention trial. The observational studies suggested that beta carotene would help prevent lung cancer in people at high risk for the disease, but the intervention study showed that beta carotene resulted in more lung cancer cases. And we certainly have plenty of these examples coming from the laboratory–a suggestion that something is bad or good but we can’t demonstrate it in the population.
But you know, I think it’s probably not useful to make the distinction between the various types of research. We actually look for the weight of the evidence. If we have good human evidence and very solid supporting experimental or laboratory evidence, we feel a lot better than if we just have good human evidence, or just good laboratory evidence. So we really look for the sum total of the relevent scientific evidence and make a causal, or at least a practical causal decision based on the sum total. And I think clinical research, basic science research, and epidemiologic research all contribute to that–the weight of the evidence.
Do epidemiologists come from a wide variety of backgrounds? What are some of the common career paths for epidemiologists?
Dr. Hoover: There’s been a change in the discipline over my career, certainly. When I was first in training, epidemiology was almost exclusively medical epidemiology and epidemiologists were physicians. And, in fact, many training programs were restricted to people with medical degrees. There’s been a very healthy trend in the discipline in the past 30 years towards taking people with a wide variety of backgrounds and teaching them the epidemiologic method and then allowing them to use their other background in concert with that method. Probably the first were the statisticians. Now we have anthropologists, we have sociologists, we have molecular scientists, we have biochemists–you name it. We even have a biophysicist-epidemiologist in our own program. It’s actually led to what I think is an extraordinarily healthy mix in the discipline. When you deal with teams, which is usual in epidemiologic research, you have complimentary training. You can have a physician-epidemiologist, a statistician-epidemiologist, and a behavioral scientist-epidemiologist working on the same project. I actually think you get a much better product that way.
What would you say are the main tasks of the epidemiologists working in your division? What takes the most time–designing studies, collecting data, or number crunching?
Dr. Hoover: Epidemiologists do all those elements. In terms of time, because epidemiological studies are typically measured in years, not weeks, you spend more time developing and monitoring field work than you do any other component. But the other components are critical to everything. You need time to develop the ideas, and find out what’s practical. Then you have to execute the study and finally make sense of it by the analysis and interpretation. When people come here as fellows, I tell them to have an eclectic research repertoire at different stages; have some studies in the field, some in the development stage, others in the analysis stage, all at the same time. This is to keep your sanity, as well as to continue the learning cycle. You do better field work when you understand the problems in the analysis that were caused by bad field work. If you know the practical constraints, you do a better job of designing the study. I encourage them to be involved in different stages and keep that mix throughout their careers.
Do people go to Iowa, for example, for three months to do a study?
Dr. Hoover: We pioneered the nationwide conduct of studies. For the most part, you travel to the site of study very intensely at the very beginning to get cooperation of the local infrastructure and the local medical establishment and to get things through committees. You also want to insure that the setting up of the field study is consistent with good research design. You set up systems that allow you to monitor the study from Bethesda. Then, throughout the study, the amount of time you spend there depends on how well the work is going. If things aren’t going well, then you’ll have to go out there. It is a constant challenge to maintain control and make sure you know what’s being done, and that it’s done well.
A lot of our work is contract-based. What we try to do is have individuals who are well-trained in field studies, contract with them to do those specific activities, and have them hire local people to do the work. That’s turned out to be the most effective way of doing the work.
It used to be that epidemiology was a cottage industry. In my training you were expected to know the literature, get the idea for study, develop the protocol, develop the questionnaire, hire interviewers, train the interviewers, supervise the interviewers, code the data, key in the data, write the programs to analyze data, and produce the manuscript. It’s probably not bad to do this in training. But it is exceptionally inefficient and probably bad to do in your career. What happens now is that the epidemiologist is the orchestrator of all this. He or she is responsible for making sure that the key elements of the design are implemented by individuals highly-trained, experienced and good at each specific task.
What does it take to be a good epidemiologist?
Dr. Hoover: Curiosity is certainly one quality, and to be intrigued by mysteries. It also helps in the daily conduct of the discipline to pay attention to detail. Sometimes it’s a difficult combination to find; you need some sort of creativity and curiosity, but you also need an extraordinary amount of discipline and attention to detail.
What training do you think is important today for young, aspiring epidemiologists? Does DCEG have an active training program?
Dr. Hoover: First and foremost, an epidemiologist needs to be well-trained in epidemiology. Epidemiology is a method, a way of thinking. It has a structure, and you don’t just do it because you have had some training in medicine or chemistry or you feel you can ask people questions. It requires good solid training in epidemiology–didactic training and then working closely with epidemiologists. And certainly that’s what we try to provide here. We have pre-doc, post-masters, post-docs in various stages of their training and can insinuate them in a variety of studies and have them work closely with other epidemiologists and people in other disciplines to get a lot of hands-on experience. That’s what’s most important. There is an increasing interest in learning molecular science, so we have a molecular epidemiology fellowship that allows people to spend time in labs. This gives them an opportunity to see what is possible and where things may go wrong. A lot of people like to sub-specialize in a particular exposure–occupational, radiation, nutrition, etc. We have training programs that allow someone to focus not only on the epidemilogic method but on the subject-matter area of interest as well.
Do you have to have mathematical aptitude to do epidemiology?
Dr. Hoover: It is useful if you’re quantitative. You don’t have to be a sophisticated statistician, but you have to appreciate the quantitative side. One of my own mentors repeatedly informed me, “if you can’t count it, you don’t know it.”
Where do graduates go after they train here?
Dr. Hoover: They go all over–to industry, academia, state governments, foreign governments, and regulatory agencies. Because you can apply the method of epidemiology to many situations, you can work in research, health care, regulation, litigation or many other areas.
Animation/Video
| This animation requires the Flash plug-in. If you do not have the plug-in, please click here to install. | |
Text Transcript
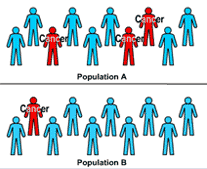
This animation depicts how odds ratios are calculated, first by comparing two populations, one of which has had an exposure to radiation and the other of which has not been exposed, then following both populations to see who gets cancer, then calculating an odds ratio based on the number of cancer cases in these two populations.
The animation opens with two populations of ten people each. Population A is exposed to I-131, a carcinogen. Population B has no exposure. Three people in Population A develop cancer, while only one person in Population B develops cancer.
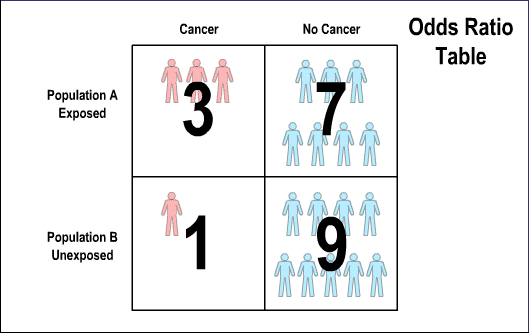
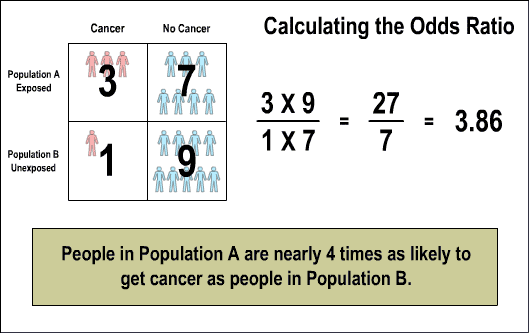
The ensuing odds ratio table breaks out the numbers: in Poplation A, on the top row of the table, three people have cancer and seven have no cancer; in Population B, on the bottom row of the table, one person has cancer and nine have no cancer. In calculating the odds ratio, three is multiplied by nine and seven is multiplied by one. Dividing out the resulting fraction, twenty-seven over seven, results in 3.86, meaning that people in Population A are almost four times as likely to develop cancer as people in Population B.
Audio Clips
- Audio clip from an interview about Epidemiology with Robert Hoover, M.D., Sc.D, NCI:
Q: Are there any fundamental differences in design between epidemiological studies focused on genetic factors and those concerned with environmental factors?( Audio – Length: 02:11 )
Text Transcript
Audio clip from an interview about Epidemiology with Robert Hoover, M.D., Sc.D, NCI:
Q: Are there any fundamental differences in design between epidemiological studies focused on genetic factors and those concerned with environmental factors?Dr. Hoover: For the most part, the methods in human populations are quite similar. There are some specific methods that are different. For example, family studies are used to identify high penetrance genes-the genes that are responsible for familial cancers. But, these kinds of studies are not very productive for studying environmental exposure because most families tend to have the same environmental exposures. Outside of that, trying to evaluate the impact of natural genetic variation in the population on cancer risk is very similar to looking at environmental exposures. It’s essentially another exposure–you are exposed to a particular version of a gene.
One difference, however, is a difference in scale. In environmental studies, we worry about confounding exposures. So, if you’re investigating coffee drinking, you have to control for the effects of tobacco because they usually correlate. But, there are usually a limited number of confounding factors that you know about or that you can assess. With the new genetic technology, there is a new possibility that if you’re interested in a particular gene, there may be thousands of others whose effects you may be called upon to control for. This does serve up a kind of challenge of scale that we haven’t seen before. In general, however, the principles are largely similar and transferable.
- Audio clip from an interview about Epidemiology with Robert Hoover, M.D., Sc.D, NCI:
Q: What are the priorities for future epidemiological studies?( Audio – Length: 01:38 )
Text Transcript
Audio clip from an interview about Epidemiology with Robert Hoover, M.D., Sc.D, NCI:
Q: What are the priorities for future epidemiological studies?Dr. Hoover: Epidemiology is an opportunistic science. It goes where the action is not only in terms of disease and exposure, but also where the tools are. Many epidemiologists are most anxious to use the new molecular tools to assess exposures better, as well as measuring susceptibility. For example, it would be wonderful to have a biological dosimeter for your exposure to benzene from gasoline fumes, or your lifetime level of consumption of fat in your diet. It’s difficult to get at these kinds of exposures by asking questions. We are hopeful that the emerging technology from measurement science will provide opportunities in this area. That is an area all of us hope will come to fruition.
Photos/Stills
1. Epidemiologists often compare two similar populations with different exposures.
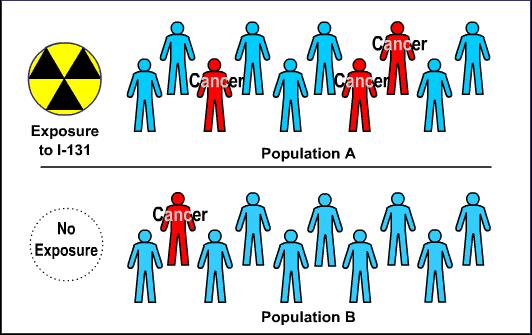
2. Odds ratio tables are a mathematical way of calculating risk in small populations.
3. Simplified calculation of risk using odds ratio.
 NCI NewsCenter
NCI NewsCenter NCI Budget Data
NCI Budget Data Visuals Online
Visuals Online NCI Fact Sheets
NCI Fact Sheets Understanding Cancer Series
Understanding Cancer Series