- Home
- Search for Research Summaries, Reviews, and Reports
EHC Component
- EPC Project
Full Report
- Research Review Jun. 1, 2012
Related Products for this Topic
- Clinician Summary Nov. 20, 2012
- Consumer Summary Nov. 20, 2012
- La Guías Sumaria de los Consumidores Feb. 7, 2013
- Executive Summary (Update) Apr. 24, 2012
Related Topics
Research Protocol – Dec. 6, 2010
Comparative Effectiveness of Drug Therapy for Psoriatic Arthritis in Adults – An Update to the 2007 Report
Formats
Table of Contents
- Background and Objectives for the Systematic Review
- The Key Questions
- Analytic Framework
- Methods
- References
- Definition of Terms
- Summary of Protocol Amendments
Background and Objectives for the Systematic Review
Psoriatic arthritis (PsA) is among the most disabling forms of arthritis, even though it affects fewer people than other types of arthritis. PsA has a highly variable presentation, which generally involves pain and inflammation in joints and progressive joint involvement and damage. The condition is associated with the skin disease psoriasis, but not all people with psoriasis will develop PsA. Additionally, PsA may predate the development of skin disease leading to some diagnostic uncertainty. Among people with psoriasis the prevalence of arthritis varies from 6% to 42%. In the general population the prevalence of PsA is estimated to be 0.3% to 1%. Based on estimates from the 2000 US Census, 520,000 persons ages 18 and above have psoriatic arthritis in the US.
Treatment of psoriatic arthritis (PsA) aims to control pain and inflammation and, ultimately, to slow the progression of joint destruction and disability. Available therapies for PsA include corticosteroids, oral disease-modifying antirheumatic drugs (DMARDs), and biologic DMARDs. The oral DMARDs used to treat patients with PsA are hydrochloroquine, leflunomide, methotrexate, and sulfasalazine. The biologic DMARDs that have been approved by the U.S. Food and Drug Administration (FDA) to treat patients with PsA are adalimumab, etanercept, golimumab, and infliximab. The biologic DMARDs are a newer category of DMARDs, which differ from conventional DMARDs in that they target specific components of the immune system.
Experts have not arrived at consensus about the comparative effectiveness of corticosteroids, oral DMARDs, and biologic DMARDs for treating PsA. More importantly, it is unclear how the effectiveness and safety of different types of combination therapy compare—e.g., oral DMARDs with corticosteroids, oral DMARDs with biologic DMARDs, triple combination of oral DMARDs, and biologic DMARDs. In addition, there is debate about how early in the disease process combination therapy should be initiated and whether patients will respond to a biologic agent if they have previously failed a different biologic agent. Many questions remain about the risks of these agents across a spectrum of adverse events from relatively minor side effects such as injection site reactions to severe and possibly life-threatening problems such as severe infections or infusion reactions. Finally, very little is known about the benefits or risks of these drugs in different patient subgroups, including ethnic minorities, the elderly, pregnant women, and patients with other comorbidities.
In patients with PsA, historically, few trials have been conducted, with only minimal research before biologic agents were introduced; management options tended to be adapted from rheumatoid arthritis (RA) trial evidence. Like in RA trials, many questions remain about the risks of these agents across a spectrum of adverse events from relatively minor side effects such as injection site reactions to severe and possibly life-threatening problems such as severe infections or infusion reactions.
This comparative effectiveness review is an update to the original one conducted in 2007 for the Agency for Healthcare Research and Quality with the same key questions (Donahue et al. 2007) but originally covering both RA and PsA in the same report. The first review did not include certolizumab pegol, golimumab, and tocilizumab drug therapies listed above, as these are new approved treatments since the time of the literature searches for the first review.
The Key Questions
KQ1: For patients with psoriatic arthritis, do drug therapies differ in their ability to reduce disease activity, to slow or limit progression of radiographic joint damage, or to maintain remission?
KQ2: For patients with psoriatic arthritis, do drug therapies differ in their ability to improve patient reported symptoms, functional capacity or quality of life?
KQ3: For patients with psoriatic arthritis, do drug therapies differ in harms, tolerability, adherence, or adverse effects?
KQ4: What are the comparative benefits and harms of drug therapies for psoriatic arthritis in subgroups of patients based on stage of disease, history of prior therapy, demographics, concomitant therapies, or comorbidities?
Changes in KQ1 and a2 wording from draft protocol
- Per suggestions from our Technical Expert Panel, the wording of KQ1 was revised to replace the words "patient reported symptoms" with "disease activity, and concurrently, "disease activity" was removed from KQ2 and replaced with "patient reported symptoms."
PICOTS criteria for the above key questions:
Population(s):
- Adults with psoriatic arthritis
- All stages of disease, history of prior therapy, demographics, concomitant therapies and comorbidities
Interventions:
Comparisons will be made between treatment strategies including any of the following FDA-approved biologic DMARDs, oral DMARDs or corticosteroids.
| Generic Name | Trade Name |
|---|---|
| Methylprednisolone | Medrol®, Depo-Medrol®, Solu-Medrol® |
| Prednisone | Deltasone®, Sterapred®, LiquiPred |
| Prednisolone | Orapred®, Pediapred®, Prelone®, Delta-Cortef®, Econopred® |
| Generic Name | Trade Name |
|---|---|
| Hydroxychloroquine | Plaquenil® |
| Leflunomide | Arava® |
| Methotrexate | Trexall®, Folex®, Rheumatrex® |
| Sulfasalazine | Azulfidine®, EN-tabs®, Sulfazine® |
| Generic Name | Trade Name |
|---|---|
| Abatacept | Orencia® |
| Adalimumab | Humira® |
| Anakinra | Kineret® |
| Etanercept | Enbrel® |
| Infliximab | Remicade® |
| Rituximab | Rituxan® |
| Certolizumab pegol | Cimzia® |
| Golimumab | Simponi® |
| Tocililizumab | Actemra®, RoActemrai® |
Comparators:
All key questions: all review drugs listed above; placebo (with biologic DMARDs only)
Outcomes for each question
- KQ 1 and KQ4:
- Progression of joint damage
- Remission
- KQ2 and KQ4:
- Physical functioning
- Quality of life
- KQ3 and KQ4:
- Morbidity, mortality and other serious adverse events
- Tolerability
- Adherence
- Other adverse effects
Timing:
Minimum of three months duration of follow-up
Settings:
Primary care and Rheumatology Specialty settings
Analytic Framework
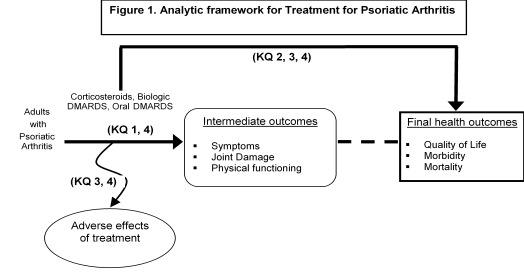
Figure 1: This figure depicts the key questions (KQs) within the context of the PICOTS described in the previous section. In general, the figure illustrates how treatment with corticosteroids, biologic DMARDs, or oral DMARDs vs. any of these same treatments may result in intermediate outcomes such as symptoms, joint damage, physical functioning, and/or long-term outcomes such as quality of life, morbidity, or mortality. Also, adverse events may occur at any point after the treatment is received.
Methods
A. Criteria for Inclusion/Exclusion of Studies in the Review
Exhibit 4-1 presents the inclusion/exclusion criteria we will use during abstract and full test review.
| Category | Criteria | |
|---|---|---|
| Inclusion | Exclusion | |
| Abbreviations: DAS = Disability Assessment Scale; HAQ = Health Assessment Questionnaire; PsARC = Psoriatic Arthritis Response Criteria; PASI = Psoriasis Area and Severity Index. | ||
| Study population | Adults (age 19 and older for PubMed) with psoriatic arthritis, all races, ethnicities, cultural groups | Children |
| Study outcomes |
|
|
| Study geography | No limits | |
| Time period | June 1, 2006 through June 30, 2009, to be updated after draft CER goes out for peer review | Prior to June 1, 2006 |
| Settings | All other settings | |
| Interventions |
|
|
| Publication language | English | All other languages |
| Admissible evidence (study design and other criteria) | Original research; eligible study designs include:
|
|
B. Searching for the Evidence: Literature Search Strategies for Identification of Relevant Studies to Answer the Key Questions
We will systematically search, review, and analyze the scientific evidence for each key question and any subsidiary questions. The steps that we will take to accomplish the literature review are described below.
To identify articles relevant to each key question, we began with a focused MEDLINE search on pharmacological treatments for rheumatoid arthritis and psoriatic arthritis using a variety of terms, MeSH and major headings, limited to English and human-only studies. We also searched other databases (The Cochrane Library and the Cochrane Central Trials Registry). Results from initial database searches are presented in Exhibit 4-2.
| #1 | Search "Arthritis, Psoriatic"[MeSH] OR "Arthritis, Rheumatoid"[MeSH] | 85528 |
| #2 | Search "Adrenal Cortex Hormones"[MeSH] OR corticosteroid* | 239378 |
| #3 | Search "Methotrexate"[MeSH] OR "leflunomide"[Substance Name] OR "Sulfasalazine"[MeSH] OR "Hydroxychloroquine"[MeSH] | 31900 |
| #4 | Search "TNFR-Fc fusion protein"[Substance Name] OR etanercept OR "infliximab"[Substance Name] OR "adalimumab"[Substance Name] OR "cytotoxic T lymphocyte-associated antigen 4-immunoglobulin"[Substance Name] OR abatacept OR remicade OR enbrel OR humira OR "rituximab"[Substance Name] OR "interleukin 1 receptor antagonist protein"[Substance Name] OR anakinra | 15567 |
| #5 | Search ((("CDP870 "[Substance Name] OR certolizumab OR cimzia) OR "efalizumab "[Substance Name] OR raptiva) OR "alefacept "[Substance Name] OR amevive) OR "natalizumab "[Substance Name] OR tysabri | 1127 |
| #6 | Search "golimumab "[Substance Name] | 12 |
| #7 | Search #2 OR #3 OR #4 OR #5 OR #6 | 283303 |
| #8 | Search #7 AND #1 | 11283 |
| #9 | Search #7 AND #1 Limits: Editorial, Letter, Practice Guideline | 1368 |
| #10 | Search #8 NOT #9 | 9915 |
| #11 | Search #8 NOT #9 Limits: Humans, English, All Adult: 19+ years | 4079 |
| #12 | Search Limits: Entrez Date from 2006/06/01, Humans, English, All Adult: 19+ years | 498212 |
| #13 | Search #11 AND #12 | 1027 |
Analogous search terms were used in other databases yielding the following results:
EMBASE: 288 (duplicates 67)
Cochrane: 15 (duplicates 0)
IPA: 142 (duplicates 10)
1395 total references imported into the “Arthritis Update July 2009” EndNote database.
| With FDA approval of tocilizumab, an additional search was conducted by using the strategy given below. | ||
| #1 | Search "Arthritis, Psoriatic"[MeSH] OR "Arthritis, Rheumatoid"[MeSH] | 85692 |
| #2 | Search actemra | 4 |
| #4 | Search "tocilizumab "[Substance Name] | 103 |
| #5 | Search #2 OR #4 | 104 |
| #6 | Search #5 AND #1 | 75 |
| #7 | Search #5 AND #1 Limits: Editorial, Letter, Practice Guideline | 8 |
| #8 | Search #6 NOT #7 | 67 |
| #9 | Search #6 NOT #7 Limits: Humans, English, All Adult: 19+ years | 14 |
| #10 | Search #9 Limits: Entrez Date from 2006/06/01 | 14 |
Of the 14 new references retrieved from PubMed, 6 were duplicates and 8 were new.
Analogous search terms were used in other databases yielding the following results:
EMBASE: 8 (6 duplicates; net of 2 added)
Cochrane: 0 reviews; 8 clinical trials (7 duplicates; net of 1 added)
IPA: 15 (6 duplicates; net of 9 added)
On August 6, 2009 an additional 20 total new references imported into the “Arthritis Update July 2009” EndNote database.
Our initial searches yielded 1,395 citations across databases. With the impending approval of tocilizumab, we conducted an additional search and added another 20 new references to the database, for a total of 1,415 citations. We will review our search strategy with the TEP and supplement it as needed according to their recommendations. In addition, to attempt to avoid retrieval bias, we will manually search the reference lists of landmark studies and background articles on this topic to look for any relevant citations that might have been missed by electronic searches. We will also conduct an updated literature search (in MEDLINE, the Cochrane Library, and the Cochrane Central Trials Registry) before completing the final draft of the report. We will be reviewing the results of a gray literature search conducted by the SRC as a means of confirming that we have captured all of the relevant studies.
C. Data Abstraction and Data Management
All titles and abstracts identified through searches against our inclusion/exclusion criteria will be independently reviewed by two trained members of the research team. Studies marked by either a reviewer as a possible include will move forward to full text review. A senior member of the team will review all excludes from both reviewers. For studies without adequate information to make the determination, we will retrieve the full text and then make the determination. All results will be tracked in the EndNote database.
For the next step, we will retrieve and review the full text of all titles included during the title/abstract review phase. Each full-text article will be independently reviewed by two trained members of the team for inclusion or exclusion based on the eligibility criteria described above. If both reviewers agree that a study does not meet the eligibility criteria, the study will be excluded. If the reviewers disagree, conflicts will be resolved by discussion and consensus or by consulting a third, independent party, usually the Principal Investigator. As above, all results will be tracked in an EndNote database including, where applicable, the reason a study did not satisfy eligibility criteria so that we can later compile a listing of excluded articles and reasons for such exclusions.
We will design data collection forms that include questions on identifying information for the article, study design, methods, and results, specifically focused on answering the key questions. Trained abstractors will extract the relevant data from each included article into the abstraction system. All abstractions are reviewed for completeness and accuracy by a second senior member of the team, most often the lead author of pertinent key question that the study pertains to.
D. Assessment of Methodological Quality of Individual Studies
To assess the quality (internal validity) of studies, we will use predefined criteria based on those developed by the US Preventive Services Task Force (ratings: good, fair, poor) and the National Health Service Centre for Reviews and Dissemination. In general terms, a “good” study has the least bias and results are considered to be valid. A “fair” study is susceptible to some bias but probably not sufficient to invalidate its results. A “poor” rating indicates significant bias (e.g., stemming from serious errors in design or analysis) that may invalidate the study’s results. To assess the quality of observational studies, we will use criteria outlined by Deeks, et al. (2003).
Two independent reviewers will assign quality ratings to each study. The first to do the quality rating is the abstractor at the conclusion of completing the abstraction. Our second quality rater is our senior reviewer, who as described above, completes a second full review of the study and is then in the best position to do an independent quality rating. Disagreements will be resolved by discussion and consensus or by consulting a third, independent party.
E. Data Synthesis
We anticipate that the data found from the literature review will be synthesized qualitatively. However, if we find a sufficient number (three or more) of similar studies of factors influencing the treatments analyzed under this review, we will consider quantitative analysis (indirect comparisons, or meta-analysis) of data from those studies.
F. Grading the Evidence for Each Key Question
We will rate the strength of evidence based on the standard methods of the EPCs, which use a revised version of the approach devised by the GRADE working group (Atkins et al. 2004). Developed to grade the quality of evidence and the strength of recommendations, this approach incorporates the following elements: study design, study quality, consistency, directness, presence of imprecise or sparse data, high probability of publication bias, and magnitude of the effect. We use four grades: high, moderate, low, and insufficient.
References
- Donahue KE, Gartlehner G, Jonas DE, Lux LJ, Thieda P, Jonas B, Hansen RA, Morgan LC, Williams SC, Lohr KN. Comparative Effectiveness of Drug Therapy for Rheumatoid Arthritis and Psoriatic Arthritis in Adults. Comparative Effectiveness Review No. 11. (Prepared by RTI-University of North Carolina Evidence-based Practice Center under Contract No. 290-02-0016.) Rockville, MD: Agency for Healthcare Research and Quality. November 2007.
- Deeks JJ, Dinnes J, D'Amico R, Sowden AJ, Sakarovitch C, Song F, et al. Evaluating nonrandomised intervention studies. Health Technology Assessment, 2003 7(27), iii-x, 1-173.
- Atkins D, Eccles M, Flottorp S, Guyatt GH et al. Systems for grading the quality of evidence and the strength of recommendations I: Critical appraisal of existing approaches: The GRADE Working Group. BMC Health Services Research 2004; 4(1): 38.
Definition of Terms
Provided within text.
Summary of Protocol Amendments
In the event of protocol amendments, the date of each amendment will be accompanied by a description of the change and the rationale.
NOTE: The following protocol elements are standard procedures for all protocols.
- Review of Key Questions
For comparative effectiveness reviews (CERs), the Key Questions are posted for public comment and finalized after the comments are reviewed. For other systematic reviews, Key Questions submitted by partners are reviewed and refined as needed by the Evidence-based Practice Center (EPC) and the Technical Expert Panel (TEP) to assure that the questions are specific and explicit about what information is to be reviewed. - Technical Expert Panel (TEP)
The TEP is selected to provide broad expertise and perspectives specific to the topic under development. Divergent and conflicted opinions are common and perceived to be healthy scientific discourse that results in a thoughtful, relevant systematic review. Therefore study questions, study designs, and/or methodological approaches do not necessarily represent the views of individual technical and content experts. The TEP provides information to the EPC to identify literature search strategies, review the draft report, and recommend approaches to specific issues as requested by the EPC. The TEP does not perform analyses of any kind nor does it contribute to the writing of the report. - Peer Review
Approximately five experts in the field will be asked to peer review the draft report and provide comments. The peer reviewers may represent stakeholder groups such as professional or advocacy organizations with knowledge of the topic. For some specific reports, such as reports requested by the Office of Medical Applications of Research at the National Institutes of Health, there may be other rules that apply regarding participation in the peer review process. Peer review comments on the preliminary draft of the report are considered by the EPC in preparation of the final draft. The synthesis of the scientific literature presented in the final report does not necessarily represent the views of individual reviewers. The dispositions of the peer review comments are documented and will, for CERs and Technical Briefs, be published 3 months after the publication of the evidence report.
It is our policy not to release the names of the peer reviewers or TEP members until the final report is published so that they can maintain their objectivity during the review process.


 E-mail Updates
E-mail Updates
