- Home
- Slides
- Home
- Tools and Resources
- Research Summaries for Consumers, Clinicians, and Policymakers
- Search for Research Summaries, Reviews, and Reports
- Research Available for Comment
- Submit a Suggestion for Research
- Submit Scientific Information Packets
- Comparative Effectiveness Research Grant and ARRA Awards
- News and Announcements
- What Is Comparative Effectiveness Research
- Who Is Involved in the Effective Health Care Program
- What Is the Effective Health Care Program
Slides
Slides: 1–12 of 87
Background: Coronary Heart Disease
Clinical Questions Addressed by the CER (1 of 2)
Presentation: Analgesics for Osteoarthritis—An Update
Keywords: comparative effectiveness | systematic review | key question
Comparative Adverse Effects of Oral Agents: CV Effects
Presentation: Analgesics for Osteoarthritis—An Update
Keywords: comparative adverse effects | outcomes | strength of evidence | NSAIDs | CV risk
Background: Prevalence
Applicability Resources
Presentation: Assessing Applicability
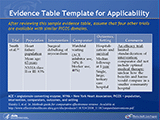
Evidence Table Template for Applicability
Presentation: Assessing Applicability
Keywords: PICOS | applicability | population | intervention | comparator | effectiveness trial | efficacy trial | PICOTS
Step 3. Completed Applicability Summary Table
Presentation: Assessing Applicability
Keywords: population | applicability | subgroup analysis | outcome | setting
Summary Table for Body of Evidence Applicability
Presentation: Assessing Applicability
Keywords: applicability | body of evidence | strength of evidence | subgroup analysis
Comparative Effectiveness of Angiotensin-Converting Enzyme Inhibitors or Angiotensin II-Receptor Blockers Added to Standard Medical Therapy for Treating Patients With Stable Ischemic Heart Disease and Preserved Left Ventricular Systolic Function
Outline of Material
Health Impact of Cardiovascular Disease in the United States (1)
Your slide tray is being processed.


 E-mail Updates
E-mail Updates