Archived Page
The information on this page is archived and provided for reference purposes only. It was current when it was produced, but may now be out-of-date. Persons having difficulty accessing this information may contact kcl@nei.nih.gov for assistance. For reliable, current information on this and other topics, we recommend that you visit the National Eye Institute website index.
Congressional Justification for FY 2007
National Eye Institute
Authorizing Legislation:
Section 301 of the Public Health Service Act, as amended.
Budget Authority:
| FY2005 Actual | FY2006 Appropriation | FY2007 Estimate | Increase or Decrease | ||||
|---|---|---|---|---|---|---|---|
| FTEs | BA | FTE | BA | FTE | BA | FTE | BA |
| 212 | $669,070,000 | 221 | $666,756,000 | 222 | $661,358,000 | 1 | $(5,398,000) |
This document provides justification for the Fiscal Year 2007 activities of the National Eye Institute, including HIV/AIDS activities. A more detailed description of NIH-wide Fiscal Year 2007 HIV/AIDS activities can be found in the NIH section entitled "Office of AIDS Research (OAR)." Detailed information on the NIH Roadmap for Medical Research may be found in the Overview section.
- Story of Discovery
- Science Advances and Future Research Directions
- NIH Roadmap
- FY 2007 Initiatives
- The NIH Neuroscience Blueprint
- Budget Policy
Introduction
Congress created the National Eye Institute (NEI) with the mission to conduct and support research, training, health information dissemination, and other programs with respect to blinding eye diseases, visual disorders, mechanisms of visual function, preservation of sight, and the special health problems and requirements of individuals who are visually impaired. Inherent in this mission is clinical research across the spectrum of diseases of the eye and disorders of vision, as well as the investigation of the normal tissue and normal visual processes that will help gain a more complete understanding of the abnormal processes that lead to these conditions. These investigations are conducted in hundreds of extramural laboratories and clinics throughout the United States and in the NEI's own intramural research facilities in Bethesda, Maryland. The highlights that follow are examples of the research progress that has been made with the investment of Federal funds in NEI-supported research and the direction that research will take over the next year.
Story of Discovery: Screening Preschoolers for Vision Disorders
In September, 1998 the Health Resources and Services Administration's Maternal and Child Health Bureau (MCHB) and the National Eye Institute (NEI) convened an expert panel to review the state-of-the-art of preschool vision screening. The expert panel was composed of pediatric ophthalmologists and optometrists, pediatricians, vision scientists, and representatives from organizations concerned with vision screening in children. Their dedication to advancing preschool vision screening was fueled by the recognition that good vision is integral to a child's lifetime health and that early detection of vision problems are thought to lead to better outcomes.
At the time of the expert panel meeting, a number of professional organizations in the United States had published policy statements advocating early vision screening along with screening guidelines. Various types of screening programs for preschool children were being implemented, but concerns remained about the scientific validity and effectiveness of these programs. Key among these concerns was to determine the best diagnostic tools and testing protocols for vision screening and to determine which age groups should be targeted.
The full meeting proceedings were published by the Department of Health and Human Services (HHS) and a summary report appeared in the journal Pediatrics in the November 2000 issue. The publications energized the preschool vision research community. One group of investigators, who had been awarded a NEI planning grant in 1996, stepped up its efforts to comprehensively evaluate preschool vision screening tests. In 2000, the Vision in Preschoolers (VIP) Study group was funded by the NEI to conduct a multi-phased, multi-center, interdisciplinary, study designed to determine whether there are tests or a combination of tests that can effectively identify preschoolers in need of a comprehensive eye exam.
Phase I of the VIP study was conducted over a two-year period during which time more than 2,500 high risk preschoolers were screened for vision disorders. Optometrists and ophthalmologists experienced in working with children administered 11 commonly used screening tests. Test results were compared to findings from comprehensive eye exams to establish their sensitivity for detecting vision disorders. The primary VIP Phase I outcome paper was published in the journal Ophthalmology in April, 2004 and was selected as the 2004 Editors' Choice award. Results showed that approximately 98 percent of 3- to 5-year-old participants could be screened successfully-an important consideration when testing preschoolers. But even in the hands of licensed eye care professionals, under controlled circumstances, the accuracy of the screening tests varied widely. In fact, a number of commonly used screening tests performed poorly. However, others performed rather well. These results provide the most comprehensive scientific evidence for evaluating preschool vision screening tests and are relevant to the professional, governmental, and private organizations concerned with children's vision.
Phase II of the VIP study was designed to assess the performance of less-skilled personnel in administering the best preschool vision screening tests as established in Phase I. Fifteen hundred high risk children were screened in typical screening environments by pediatric nurses and lay screeners. Comparison of screening results to findings from a comprehensive eye examination indicated that specially trained nurses and lay people can achieve results that are comparable to screenings performed by licensed eye care professionals. VIP Phase II results were published in the August 2005 issue of Investigative Ophthalmology and Visual Science and provide scientific evidence that non-professionals can perform select tests with good accuracy.
While the results of VIP Phase I and II provide rigorous data with regard to some aspects of preschool vision screening, key issues remain to be addressed. Anecdotal reports indicate that only a fraction of U.S. children who fail a screening exam go on to receive a comprehensive eye exam and needed treatment. Thus, keen interest lies in research designed to maximize screening follow-up. And while there is scientific evidence from a small number of studies conducted outside the U.S. indicating that preschool vision screening reduces the prevalence of vision disorders among school-age children, there is little evidence quantifying screening's impact on children's educational achievement and functional status. NEI will continue to support high quality studies to address these issues.
Science Advances and Future Research Directions
Retinal Diseases
The retina is the complex, light-sensitive, neural tissue in the back of the eye that contains highly-specialized and metabolically active photoreceptor cells (rods and cones). These cells respond to light by emitting chemical and electrical signals. The signals are received by other retinal cells that process and transmit visual information via the optic nerve to the brain for further processing. The choroid is the underlying layer of blood vessels that nourish the retina. The retina and choroid are susceptible to a variety of diseases that can lead to visual loss or complete blindness. These sight-threatening conditions include age-related macular degeneration, diabetic retinopathy, retinopathy of prematurity, retinitis pigmentosa, Usher's syndrome, ocular albinism, retinal detachment, uveitis (inflammation), and cancer (choroidal melanoma and retinoblastoma).
Gene discovery for age-related macular degeneration: Late onset degenerative diseases like age-related macular degeneration (AMD) are thought to result from the confluence of genetic predisposition and chronic exposure to environmental risk factors. In this scenario, a gene or genes contain common but subtle variations, known scientifically as polymorphisms, that hamper cellular function but may not result in disease. However, years of environmental insult, such as cigarette smoking, poor diet, and hypertension, further strain the underlying genetic burden to a tipping point that provokes outright disease. On the genetic side of the equation, identifying polymorphisms in AMD and other late onset diseases has been complicated by the fact that traditional genetic research strategies and tools are either inadequate or too cumbersome in their application. The development of more sophisticated genetic tools, such as the International HapMap Project has enabled scientists to scan the entire human genome more quickly and efficiently. Using data from the International HapMap project, four different NEI supported laboratories identified a common variation in a gene called complement factor H (CFH) that accounts for as many as 50 percent of AMD cases. The CFH protein regulates an inflammatory response that is typically triggered by infectious microbes. However, alterations in the CFH gene are postulated to poorly regulate this response, leading to chronic, localized inflammation and ensuing damage to cells in the center of the retina, the macula, and its neighboring tissues. Inflammation is thought to play a role in many common diseases such as Alzheimer's disease, Parkinson's disease, multiple sclerosis, stroke, and atherosclerosis. Although the cells, tissues, and molecular events in these diseases are diverse, they may share some common disease mechanisms that present an opportunity to cross pollinate findings from diverse research areas. Discovery of the CFH gene will make it possible to develop new animal models for AMD and evaluate therapies that control or minimize chronic inflammation.
New mouse model for macular degeneration: Stargardt-like macular dystrophy (STGD3) is a rare, inherited form of juvenile macular degeneration that shares many clinical features with AMD, including the accumulation of deposits called lipofuscin in the retina prior to the degeneration of the photoreceptor cells. As of yet, there are no suitable animal models for AMD, and so the development of a rodent model for STGD3 may offer insights into both diseases. In 2001, alterations in a gene called ELOVL4 were found to cause STGD3. This year, vision researchers developed a transgenic mouse model with a mutant form of the ELOVL4 gene. The mutant ELOVL4 mice were found to possess some of the hallmarks of macular degeneration, including accumulation of lipofuscin in the retina, abnormal neural activity and localized degeneration of photoreceptor cells in the retina. The availability of the ELOVL4-mutant mouse will facilitate our understanding of the basic pathogenesis of macular degeneration and offer a model to evaluate therapeutic interventions.
RPE65 and the visual cycle: Vitamin A and its derivatives are critical components to vision. In photoreceptor cells, a vitamin A derivative, called 11-cis-retinaldehyde, combines with a protein called opsin to form rhodopsin. Rhodopsin is the key molecule that absorbs light and begins the cascade of molecular events that converts light to the chemical and electrical signals that our brains process to visualize our surroundings. When light hits rhodopsin, 11-cis-retinaldehyde is changed to another vitamin A derivative called all-trans-retinaldehyde. However, once rhodopsin is converted to this form, it can no longer absorb light. Through a process called the visual cycle, 11-cis-retinaldehyde is renewed so that it can again participate in the visual process. Studying the gene expression underlying the visual cycle, scientists discovered that a previously known protein called RPE65 is responsible for the chemical conversion of spent rhodopsin. Mutations in the RPE65 gene are known to cause a range of retinal degenerative diseases that vary widely in severity. For example, some mutations are associated with Leber's congenital amaurosis, an eye disease that causes blindness in infants, while others result in mild to moderate forms of retinitis pigmentosa. A more precise understanding of RPE65 will help clarify our knowledge of the visual cycle and the diverse diseases that emerge from alterations in this gene.
LRAT and the visual cycle: Building on the study described above, scientists used an artificial visual cycle developed in cell culture to study RPE65 function. Importantly, they found that although RPE65 is the only protein product needed for renewal of 11-cis-retinaldehyde, robust production of this vitamin A derivative relies on an enzyme called lecithin retinol acyltransferase (LRAT). Previous studies have found that mutations in the gene that encodes LRAT also cause a severe, early onset retinal degenerative disease. LRAT was found to provide the immediate precursor to 11-cis vitamin A and induce RPE65 activity. Results from the cell culture study also revealed that an iron atom was required in the renewal process, establishing the previously unappreciated role of iron in the visual cycle. In total, these two studies confirm that RPE65 is essential to the visual cycle and that LRAT acts in concert with this protein. Gene therapy trials for blindness due to mutations in RPE65 have been successful in restoring vision in mice and dogs lacking RPE65 and are now planned for humans. The new results confirm that RPE65 gene therapy directly targets the key component of the defective process causing this form of blindness.
An improved understanding of uveitis: Uveitis is the name given to a group of blinding, inflammatory eye diseases that are often autoimmune in nature. A collaborative team of researchers has found new evidence that the thymus gland plays a critical role in the development of uveitis. Located in the chest cavity, the thymus acts as a programming center for the immune system. Immature lymphocytes created from bone marrow stem cells enter the thymus and develop into a wide variety of T cells (T stands for thymus) that have affinity for infectious agents that invade the body. The thymus also contains a catalog of tissue-specific self-antigens. Self-antigens are those cell elements that hold potential to initiate an autoimmune response. The developing T cells that have affinity for these self-antigens are eliminated in the thymus, thus creating tissue tolerance and preventing the potential for autoimmune diseases. In the current study, NEI scientists found that human thymus samples contained several eye-related self-antigens that have been previously associated with the development of uveitis in animal models. However, the expression of these tissue antigens varied remarkably among the individual samples. Previous studies have found evidence that susceptibility to autoimmune disease decreases when the level of expression of a self-antigen within the thymus is robust. These findings greatly clarify the mechanisms that determine the susceptibility to uveitis. The discovery that the thymus plays a role in the disease also opens a new avenue of investigation to expand our understanding of uveitis and to develop therapies that prevent the disease or limit vision loss.
Corneal Diseases
The cornea is the transparent tissue at the front of the eye that serves two specialized functions. The cornea forms a protective physical barrier that shields the eye from the external environment. It also serves as the main refractive element of the eye, directing incoming light onto the lens. Refraction depends on the cornea acquiring transparency during development and maintaining this transparency throughout adult life. Refractive errors such as nearsightedness (myopia), farsightedness (hyperopia) and astigmatism are the most common causes of correctable visual impairment. Corneal disease and injuries are some of the most painful ocular disorders.
Anti-inflammatory molecules in cornea wound healing: Inflammation is a common immune response to injury and infection in the body. In the cornea, however, inflammation can cause extreme discomfort and result in vision loss. Nonetheless, the cornea retains a remarkable capacity for wound repair while actively suppressing an inflammatory response. Scientists have recently discovered that two lipids, lipoxin A4 (LXA4) and docosahexaenoic acid-derived neuroprotectin D1 (NPD1), are formed in the cornea and act as anti-inflammatory agents during corneal infection and wound healing. Topical treatment with LXA4 and NPD1 in mice with corneal injuries increased the rate of tissue repair and inhibited inflammation without impairing the recruitment of key immune leukocytes, which are normally associated with inflammation, into the wounded tissue. Moreover, a transgenic mouse that lacks these lipids exhibited delayed wound healing and attenuated leukocyte recruitment. The identification of these anti-inflammatory lipids in the cornea and their enhancement of wound healing by topical application suggest their use as therapeutic agents to overcome aberrant and damaging inflammatory responses in the eye.
A role for chromosomal protein HMGN1 in corneal maturation: Corneal diseases are plentiful and difficult to treat. For example, keratoconus is a progressive corneal disease that results in a thin, bulging, conically shaped cornea that can cause severe visual impairment. Other corneal diseases are associated with blistering and poor adhesion of corneal epithelial cells, the outermost cell layer of the cornea. Many of these diseases progress to a point where the cornea is no longer viable. However, unlike intraocular lens implants to treat cataracts, corneal implants are much less successful and may become opaque with time. There are also no artificial corneas available yet. Thus, knowledge of the mechanisms regulating the development and maintenance of the cornea is of medical importance. Previous studies have shown that p63, a transcription factor involved in growth and specialization of epithelial cells, is expressed in cornea cells. Transcription factors are proteins that recognize and bind to specific regions of a gene that regulate its expression or activity. A recent study has found that during development corneal cells also express a protein called high mobility group protein N1 (HMGN1). Both p63 and HMGN1 are expressed in precisely the same set of corneal cells with a pattern that changes during corneal cell development. Loss of HMGN1 results in corneal epithelium thinning, loss of proper stratification of corneal cell types, blistering, and an abnormal rate of growth of epithelial cells. Moreover, cornea lacking HMGN1 show premature changes in p63 expression. The finding that p63 and HMGN1 are connected with aspects of corneal biology provides new targets for investigations that may ultimately have clinical relevance.
Gene discovery for corneal dystrophy: Francois-Neetens mouchetée fleck corneal dystrophy (CFD) is a rare genetic corneal dystrophy characterized by numerous small white flecks scattered in the cornea. Vision researchers have identified mutations in a gene called phosphatidylinositol-3-phosphate 5-kinase, type III (PIP5K3) that cause CFD. PIP5K3 is part of a family of enzymes that help regulate the formation and intracellular location of lipid products. It is thought that mutations in the gene disrupt the transport of lipids within the membranes of corneal cells, resulting in the characteristic flecks that appear in the corneas of people with CFD. These flecks are thought to be lipid deposits. Besides providing insight into the pathophysiology of CFD, this discovery provides a new avenue of exploration into both corneal biochemistry and physiology.
Lens and Cataract
Cataract, an opacity of the lens of the eye, interferes with vision and is the leading cause of blindness in developing countries. In the U.S., cataract is also a major public health problem. An estimated 26.6 million Americans over age 40 have cataract or have had surgery to remove the lens opacification. Currently, cataract surgery accounts for 60 percent of vision-related Medicare expenditures. However, by 2020 researchers estimate that 39.6 million Americans will be affected by cataract1. The enormous economic burden of cataract will only worsen as the American population ages. The major goals of this program, therefore, are to determine the causes and mechanisms of cataract formation, to search for ways to slow or prevent the progression of cataract, and to develop and evaluate new diagnostic and therapeutic techniques in cataract management.
Lens Borrows Cell Death Enzymes to Maintain Transparency: The lens is a dense, compact structure containing two cell types: metabolically active epithelial cells and quiescent fiber cells. Throughout the life-time of an individual, the lens carries out a process of continued growth with epithelial cells dividing and differentiating into fiber cells. During this process, the emerging fiber cells become denuded of organelles such as the nucleus and mitochondria. This process in part helps the lens achieve the high transparency needed for clear vision. Scientists have previously found that the lens uses proteins involved in a biological process called apoptosis or programmed cell death to rid lens fiber cells of their organelles. This past year, vision researchers have discovered a process that allows these apoptotic processes to occur without causing the death of fiber cells. They have termed the process, Apoptosis-related Bcl-2 and Caspase-dependent (ABC) differentiation. In this process, a number of proteins that normally lead to cell death such as caspases-proteins that break-down internal cellular structures-are used to signal the beginning of differentiation. The expression of cell death proteins is balanced by the simultaneous induction of pro-survival molecules such as bcl-2, a protein that binds to cell death proteins and inhibits further damage or death to fiber cells. This allows lens fiber cells to achieve the high level of transparency needed for clear vision. The discovery of ABC differentiation in the lens will allow researchers to better understand lens cell renewal and determine whether faulty mechanisms in this process might lead to cataract formation.
Vascular remodeling in the lens: During development, the immature lens and pupillary membrane are nourished by blood vessels that regress as the eye matures. However, in some cases, these vessels fail to regress, causing persistent fetal vasculature syndrome (PFVS), a condition that leaves the lens opaque. A recent study of patients with PFVS found persistent expression of vascular endothelial growth factor (VEGF), a protein associated with blood vessel growth. The study also found evidence to suggest that beta and gamma crystallin, two common lens proteins that help give this tissue its transparency, function outside the lens to create and maintain these developmental vessels. This is the first time that these non-lens functions have been suggested for beta and gamma crystallin. Further study is needed to determine whether non-lens beta and gamma crystallin are important in the pathogenesis of PFVS and other diseases.
Lens development and retina: The development of the human eye requires that diverse tissues differentiate and migrate in a coordinated fashion. Researchers have been unclear how this developmental coordination is achieved. A recent study has found that the PITX3 gene, which is associated with a variety of congenital eye diseases that affect the lens and cornea, is involved in coordinated development of the lens and retina. Investigators prevented expression of the PITX3 gene in zebrafish and found abnormalities in lens and retinal development. Retinas of these fish had degenerated cellular nuclei and fewer neuronal cells, suggesting that this lens protein exerts influence on retinal cell differentiation and development. This study provides a new animal model to further understand the developmental pathways that coordinate diverse tissue development within the eye.
Glaucoma and Optic Neuropathies
Glaucoma is a group of eye disorders that share a distinct type of optic nerve damage, which can lead to blindness. Elevated intraocular pressure (pressure inside the eye) is frequently, but not always, associated with glaucoma. Glaucoma is a major public health problem and the number one cause of blindness in African Americans. Approximately 2.2 million Americans have been diagnosed with glaucoma and the prevalence of the disease will rise to a projected 3 million by 20202. Most of these cases can be attributed to primary open angle glaucoma, an age-related form of the disease. NEI activities in glaucoma research are directed toward understanding the mechanisms of the disease, identifying risk factors, and preventing blindness.
Neuroprotection in glaucoma: The defining event that leads to vision loss in all forms of glaucoma is the degeneration of retinal ganglion cells (RGC) in the back of the eye. These cells relay visual information to the brain through the optic nerve and their loss effectively severs the neural network that allows us to process visual information. However, little is known about the molecular pathways that result in RGC degeneration. Using high dose radiation and bone marrow rescue to explore inflammatory responses in an animal model of glaucoma, researchers unexpectedly discovered that this procedure prevents the loss of RGCs. The neuroprotection offered by this procedure was complete, highly reproducible, and lasting. Normally, by 12-14 months, these glaucoma susceptible mice have complete RGC loss. At 14 months, treated mice had no detectable signs of disease. Although the mechanism that offers neuroprotection is not yet known, researchers speculate that it is due to radiation, because the transferred bone marrow was genetically identical to the original bone marrow the mice were born with. This highly novel treatment protocol offers a tool to understand neurodegeneration and, with refinement, could have important implications for the treatment and prevention of neurodegenerative diseases.
Myocilin and glaucoma: Myocilin is a protein of considerable interest in glaucoma research. Previous studies have found that the myocilin gene is expressed in the cells of the trabecular meshwork (TM). The TM is involved in regulating the outflow of aqueous humor, the watery fluid between the cornea and the iris at the front of the eye. Mutations in the myocilin gene were previously found to cause a rare, inherited, early onset form of glaucoma. It is thought that mutated myocilin interferes with the passage of aqueous humor in the TM, thus increasing intraocular pressure (IOP). Additionally, patients with common forms of glaucoma have increased levels of the normal myocilin protein in the TM. Long-term treatment with steroids, which is known to increase IOP and lead to glaucoma, is also thought to increase the production of myocilin. Lastly, organ culture studies with human trabecular meshwork have suggested that myocilin may impede outflow of aqueous humor. In order to study the consequence of increased myocilin in vivo, scientists developed a mouse model genetically engineered to secrete large quantities of human myocilin into the aqueous humor. Surprisingly, IOP did not increase despite the fact that levels of myocilin in their aqueous humor were five times that in the normal human eye. In parallel experiments, mice were genetically engineered to express a mutated form of myocilin. The mutated protein product caused a detrimental accumulation in the cells of the TM and was not found in aqueous humor. This study has documented that high levels of the normal myocilin protein in the aqueous humor do not necessarily produce glaucoma. Conversely, the finding that mutated myocilin accumulates within the TM, offers an explanation for the difficulty these cells have in regulating IOP. This accumulation is thought to be responsible for the subsequent development of glaucoma.
Epidemiologic findings in glaucoma: Glaucoma is thought to result from a complex interaction of chronic environmental exposure to various risk factors and a multigenic predisposition to the disease. Identifying the genetic basis of complex eye diseases like glaucoma is a major research goal of the NEI. Because increased IOP is often associated with the development of glaucoma, investigators with the Beaver Dam Eye Study (BDES) performed a complex analysis of 2337 individuals from 620 families to determine whether a genetic predisposition to increased IOP exists. The BDES is a long-term prospective epidemiologic study of a large population in Beaver Dam, Wisconsin designed to collect information about age-related eye diseases. The study authors found that increased IOP correlates with multigenic and environmental influences. The authors also found two new genetic loci on chromosomes 6 and 13. This study confirms the multigenic nature of glaucoma and suggests new areas of investigation to identify the genes that confer risk to the disease. In a parallel study that examined possible environmental findings, the BDES investigators found significant, direct correlations between elevated blood pressure and elevated IOP. Although further investigation is needed, this finding suggests the possibility that treatment to lower blood pressure might also reduce the risk of developing glaucoma. In tandem, these studies add to our understanding of environmental and genetic risk factors for glaucoma.
Strabismus, Amblyopia, and Visual Processing
Developmental disorders such as strabismus (misalignment of the eyes) and amblyopia (commonly known as "lazy eye") affect 2-4 percent of the U.S. population3,4. The correction of strabismus is one of the most frequently-performed ophthalmic surgical procedures. In addition to research relevant to strabismus and amblyopia, the NEI supports investigations of irregular eye movements and refractive errors. Three million Americans now have low vision, a term used to describe chronic visual conditions that are not correctable by eye glasses or contact lenses. The NEI also supports research on improving the quality of life of persons with visual impairments by helping them maximize the use of remaining vision and by devising aids to assist those without useful vision.
Seeing the Brain Seeing: A long sought goal in neuroscience has been to visualize large assemblies of living nerve cells in the brain while they are active with sub-millisecond time resolution. However, imaging of the cerebral cortex has been limited to observing single neurons painstakingly labeled with calcium indicators that signal physiological activity. A recent advance in cell labeling now makes it possible to study the functional activity of thousands of neurons at once. Combining this labeling technique with a relatively new imaging technology called two-photon microscopy, vision researchers have developed a powerful new approach to visualize the structure and function of neural tissue. This approach offers great sensitivity, allowing researchers to see the waxing and waning of discrete neuronal responses that code for visual stimuli. One can examine all the cells present at one depth or create three-dimensional maps by imaging the cortex at multiple depths. This approach provides an unprecedented insight into the functional organization of how we process sensory information. Gaining an understanding of the circuits underlying cortical function is possibly one of the most difficult and important challenges in neuroscience. This knowledge holds great clinical importance as well. The neurological and psychiatric diseases with perhaps the largest impact on public health-Alzheimer's disease, stroke, epilepsy, depression, and schizophrenia-are all disorders of cortical function. The application of two-photon microscopy makes it possible to study the entire cerebral cortex with unprecedented detail. Combined with advances in functional neural staining, it should also become the simplest and least invasive way to study physiological processes in normal brains and in models of neurological disease.
Brain Mechanisms of Visual Attention: As we look out our window, we think we see the whole scene but actually we do not. Instead our eyes move in rapid, successive steps, called saccades, from one part of the scene to another so that we see successive little snapshots instead of the whole picture. However, experiments have shown that attention to specific features in the visual scene is critical for our stable perception despite these continual image shifts. Therefore, saccadic eye movements would seem to undermine visual stability because they move the image falling on the retina several times per second. Researchers have hypothesized that a region of the brain, called the superior colliculus, which generates saccadic eye movements, also contributes to the directed attention necessary for enhanced visual processing. In designing experiments to uncover the processes underlying shifts in visual attention, researchers developed a task in which monkeys have great difficulty seeing dramatic changes in a visual scene unless the animal's attention is drawn to the change. They next introduced the monkeys to a visual scene and stimulated the superior colliculus with weak electric currents in order to artificially direct attention to the change. The researchers found that activation of the superior colliculus enabled the monkeys to see the change just as if the animals had attended to it on their own. In this way, researchers could mimic shifts of visual attention by direct activation of the brain. These experiments therefore show that the superior colliculus both generates saccadic eye movements and directs visual attention to details within the visual field. This study provides a first step towards understanding the circuits in the brain that underlie visual attention.
Impaired Sight and the Brain: Macular degeneration (MD) can damage the central portion of the retina, known as the macula, leaving patients legally blind and unable to perform everyday tasks such as driving, reading, and recognizing faces. Until now it has been unclear how the part of the brain that processes central vision is affected by the lack of input from the retina. To better understand this question, researchers studied the visual cortex of patients who had MD for more than 20 years using a non-invasive imaging technique known as functional magnetic resonance imaging. Despite the extensive damage to the central retina, the investigators found that the part of the brain that would normally only respond to central visual information was now responding to peripheral visual information. These findings indicate that the visual cortex in adults is capable of functional plasticity. The issue of plasticity poses many new questions that are important in developing improved rehabilitation strategies, or in creating therapies and prosthetic devices that restore central visual function. For example, do patients with MD develop better peripheral vision than normally sighted people? How quickly in the disease process does this brain reorganization occur? Would a reorganized visual cortex be capable of once again processing central vision? What are the biological mechanisms that promote these changes? Answers to these questions will lead to a better understanding of how modifiable the human brain is, and will give greater insight into the consequences of MD and the prospects for rehabilitation and treatment.
NIH Roadmap
A goal of the NIH Roadmap Nanomedicine Initiative is to characterize quantitatively the molecular scale components or nanomachinery of cells and to precisely control and manipulate these molecules and supramolecular assemblies in living cells to improve human health. The NEI has a leadership role in implementing the NIH Roadmap Nanomedicine Initiative. Under this initiative, a request for application was prepared to award 20 Nanomedicine Center Concept Development Awards. These concept development awards were created to allow applicants time and resources to develop the concept for a Nanomedicine Center that would address various issues in nanomedicine including, biomolecular dynamics, intracellular transport, and protein-protein interactions. The Centers will also determine what additional measurements and analytical and computational tools are needed to understand biological system design at the molecular level. Next, the Centers will develop, refine, and apply these tools to biological systems. This, in turn, will lead to using the knowledge to engineer molecular structures, assemblies, and organelles for treating diseased or damaged cells and tissues. It is anticipated that reaching all of these goals may require ten years or more. Four Nanomedicine Centers were awarded in FY 2005. The Centers will be dedicated to understanding the nanobiology that underlies protein folding machinery; ion channels and ion transport proteins; synthetic signaling and motility systems; and mechanical biology. The NIH expects to issue another RFA in FY 2006 to fund additional Nanomedicine Centers. The Nanomedicine Initiative will also benefit eye research in a more direct way. Current NEI grantees are exploring the use of nanotechnology to assist in corneal wound healing and drug delivery to the retina. Increased support of nanomedicine through the NIH Roadmap will undoubtedly speed progress in these areas.
FY 2007 Initiatives
Age-Related Eye Disease Study II: The Age-Related Eye Disease Study II (AREDS II), a new intramural study supported primarily with R&D contract funds, was initiated in late 2005 and will be expanded in FY 2007. The primary objective of AREDS II is to determine whether oral supplementation with lutein and zeaxanthin and/or omega-3 long-chain polyunsaturated fatty acids (LCPUFAs) will decrease the progression to advanced age-related macular degeneration (AMD). The effect of lutein and/or omega-3 LCPUFAs on the incidence of cataract surgery will also be measured. The original AREDS evaluated vitamins C, E, beta-carotene and zinc with copper for the treatment of AMD and age-related cataract. Treatment with the combination of antioxidant vitamins and minerals resulted in a 25% reduction in the development of advanced AMD at 5 years. Data from AREDS and other epidemiologic studies provided supporting evidence to consider a clinical trial to evaluate the potential therapeutic effects of lutein, zeaxanthin and omega-3 LCPUFAs. This study will be conducted in 20 to 70 clinical centers throughout the nation with a centralized data coordinating center and a fundus photograph reading center. This study will also address the NIH Roadmap initiative of re-engineering the clinical research enterprise by establishing partnerships with community-based physicians as well as clinical researchers from academic centers. During its initial years, the study activities and associated costs will be high as AREDS II investigators recruit and follow the eligible patients in this randomized, controlled clinical trial. This plan is to follow all patients for at least 5 years.
Inflammation in Degenerative Eye Diseases: The objective of this initiative is to investigate the role of inflammation in degenerative eye diseases such as age-related macular degeneration (AMD), diabetic retinopathy, uveitis; and other chronic disorders of the eye. Inflammation is the body's first line of host (innate) immune response to foreign challenge or tissue injury, and provides the necessary signals to instruct the body to mount an adaptive immune response, ultimately restoring the tissue structure and function to health. Prolonged inflammation can, however, cease to be a beneficial event and instead contribute to the pathogenesis of many disease states. Insights into the etiology of degenerative eye diseases have been slow in development because of their late onset. Using genomic approaches, mutations in the complement factor H gene, a protein involved in regulating inflammatory responses, was recently linked to AMD. There is also a dramatic increase in the understanding of the vital role of inflammation in many diseases such as cancer, diabetes, arthritis, Alzheimer's disease and atherosclerosis. This initiative will have an emphasis on using our latest knowledge of the molecular and cellular aspects of inflammation to study the development and progression of degenerative eye diseases. A second emphasis is to expand our knowledge in how the inflammatory process is kept under tight control using the eye as a model system. The knowledge gained will be pivotal to the development of new diagnostic and intervention strategies to halt and reverse the progression of degenerative eye diseases.
The Role of Cdk5 in Maintaining the Integrity of the Corneal Epithelial Cell Sheet: Cell-cell adhesion is critically important to epithelial cells of all tissues, allowing them to form tight boundaries between external and internal compartments. In the cornea, the epithelial barrier is the principal defense against invasion by infectious organisms. Recently, researchers have observed that inhibiting the activity of an enzyme called Cdk5 in migrating corneal epithelial cell sheets causes cells to separate from one another, disrupting the integrity of the cell sheet. This observation suggested a novel role for Cdk5 as a regulator of corneal epithelial cell-cell adhesion. In view of the physiological importance of epithelial cell-cell adhesion, NEI intramural researchers have undertaken an initiative to explore the molecular basis for this effect. This study will examine the location, transport, and activation of Cdk5 in epithelial cells of the cornea. The results of this study are expected to reveal novel regulatory pathways responsible for maintaining the integrity of the epithelial cell sheet.
NIH Neuroscience Blueprint
The NIH Neuroscience Blueprint is a collaborative effort among 15 NIH institutes and centers to accelerate the pace of discovery and understanding in neurosciences research. In an effort to better understand all elements of the nervous system, the Blueprint will focus on the development of tools and resources that will facilitate research on the processes of development, neurodegeneration, and plasticity that underlie the health and disorders of the nervous system. One of the approaches to develop these tools and resources is a cellular level approach to discovering the key molecules involved in nervous system function. There is still a need to identify the location, the developmental timing, and the cellular function of most of the genes and proteins expressed in the brain. Mapping of the neurogenome is being conducted by creating and analyzing transgenic mice to map gene expression and activity to different cell types and regions of the mouse central nervous system. The NEI component of this effort will be to ensure that the genes involved in neurons of the complete visual system are included in the neurogenome map.
Budget Policy
The Fiscal Year 2007 budget request for the NEI is $661,358,000, a decrease of $5,398,000 and 0.8 percent over the FY 2006 Appropriation. Included in the FY 2007 request is NEI's support for the trans-NIH Roadmap initiatives, estimated at 1.2% of the FY 2007 budget request. A full description of this trans-NIH program may be found in the NIH Overview.
A five year history of FTEs and Funding Levels for NEI are shown in the graphs below. Note that as the result of several administrative restructurings in recent years, FTE data is non-comparable.
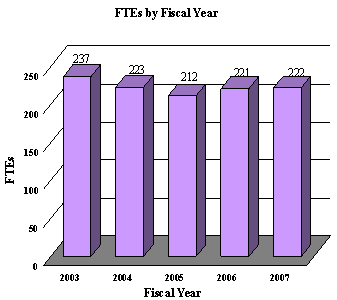
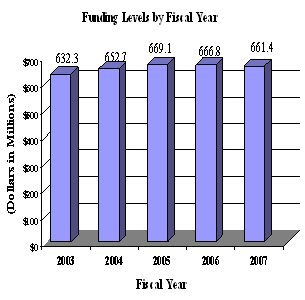
NIH's highest priority is the funding of medical research through research project grants (RPGs). Support for RPGs allows NIH to sustain the scientific momentum of investigator-initiated research while pursuing new research opportunities. We estimate that the average cost of competing RPGs will be $345,000 in FY 2007. While no inflationary increases are provided for direct recurring costs in noncompeting RPGs, where the NEI has committed to a programmatic increase for an award, such increases will be provided.
NIH must nurture a vibrant, creative research workforce, including sufficient numbers of new investigators with new ideas and new skills. In the FY 2007 budget request for NEI, $360,000 will be used to support 4 awards for the new K/R "Pathway to Independence" program.
NEI will also support the Genes, Environment, and Health Initiative (GEHI) to: 1) accelerate discovery of the major genetic factors associated with diseases that have a substantial public health impact; and 2) accelerate the development of innovative technologies and tools to measure dietary intake, physical activity, and environmental exposures, and to determine an individual's biological response to those influences. The FY 2007 request includes $1,130,000 to support this project.
In the FY 2007 request, stipend levels for trainees supported through the Ruth L. Kirschstein National Research Service Awards will remain at the FY 2006 levels.
The FY 2007 request includes funding for 44 research centers, 165 other research grants, including 69 career awards, and 64 R&D contracts. Intramural Research decreases by 0.5 percent. Research Management and Support increases by 1.5 percent.
The mechanism distribution by dollars and percent change are displayed below:


SIGNIFICANT ITEMS IN HOUSE, SENATE, AND CONFERENCE APPROPRIATIONS COMMITTEE REPORTS
1 Prevalence of cataract and pseudophakia/aphakia among adults in the United States. Arch Ophthalmol 122: 487-494, 2004.
2 Prevalence of open-angle glaucoma among adults in the United States. Arch Ophthalmol 122: 532-538, 2004.
3 The evolving concept of amblyopia: a challenge to epidemiologists. Am J Epidemiol 118(2): 192-205, 1983.
4 Baltimore Vision Screening Project. Ophthalmology 103(1): 105-109, 1996.

