Gene Therapy for Leber Congenital Amaurosis
NEI Funding of Basic and Preclinical Studies Prior to the LCA Gene Therapy Trial
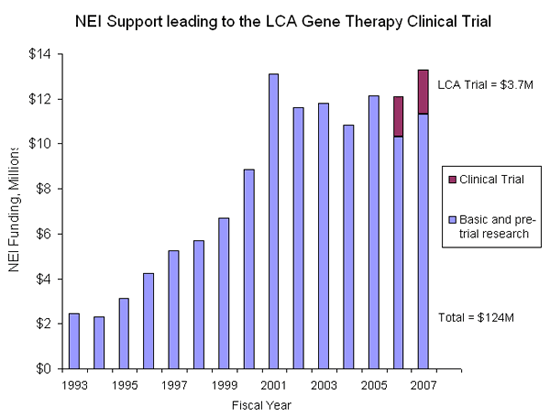
NEI support leading to RPE65-LCA gene therapy clinical trials consisted of significant efforts by investigators in the intramural laboratories at the NEI as well as many extramural investigators at universities funded by NEI grants.
The NEI spent $124M, over a 15 year period (1993 to 2007), since the RPE65 gene was identified. Areas of study included basic science studies of the gene and vector development, observational clinical research of various forms of LCA, development and testing of animal models (rodents and dogs), genetics, pathophysiology of LCA, and gene therapy in animal models.
NEI support of ancillary studies in these topical areas is not included. For example, only support of AAV vector development specifically targeted for use in RPE65 studies was included.