About BSE

Beef cattle grazing. (Image courtesy USDA)
BSE (bovine spongiform encephalopathy) is a progressive neurological disorder of cattle that results from infection by an unusual transmissible agent called a prion. The nature of the transmissible agent is not well understood. Currently, the most accepted theory is that the agent is a modified form of a normal protein known as prion protein. For reasons that are not yet understood, the normal prion protein changes into a pathogenic (harmful) form that then damages the central nervous system of cattle.
Research indicates that the first probable infections of BSE in cows occurred during the 1970's with two cases of BSE being identified in 1986. BSE possibly originated as a result of feeding cattle meat-and-bone meal that contained BSE-infected products from a spontaneously occurring case of BSE or scrapie-infected sheep products. Scrapie is a prion disease of sheep. There is strong evidence and general agreement that the outbreak was then amplified and spread throughout the United Kingdom cattle industry by feeding rendered, prion-infected, bovine meat-and-bone meal to young calves.
The BSE epizootic in the United Kingdom peaked in January 1993 at almost 1,000 new cases per week. Over the next 17 years, the annual numbers of BSE cases has dropped sharply; 14,562 cases in 1995, 1,443 in 2000, 225 in 2005 and 11 cases in 2010. Cumulatively, through the end of 2010, more than 184,500 cases of BSE had been confirmed in the United Kingdom alone in more than 35,000 herds.
There exists strong epidemiologic and laboratory evidence for a causal association between a new human prion disease called variant Creutzfeldt-Jakob disease (vCJD) that was first reported from the United Kingdom in 1996 and the BSE outbreak in cattle. The interval between the most likely period for the initial extended exposure of the population to potentially BSE-contaminated food (1984-1986) and the onset of initial variant CJD cases (1994-1996) is consistent with known incubation periods for the human forms of prion disease.
Overview of BSE in North America
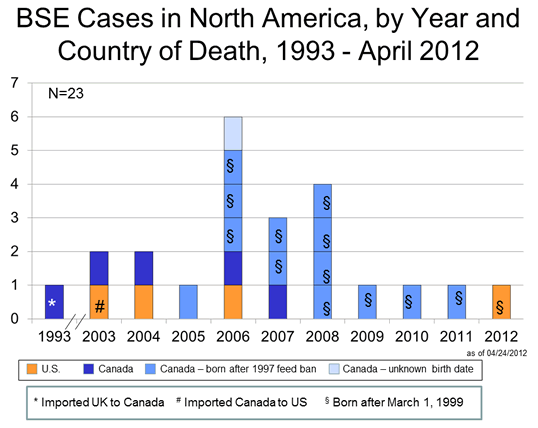
Click to enlarge
Through April 2012, BSE surveillance has identified 23 cases in North America: 4 BSE cases in the United States and 19 in Canada. Of the 4 cases identified in the United States, one was born in Canada; of the 19 cases identified in Canada, one was imported from the United Kingdom (see figure above).
BSE Cases Identified in the United States
There have been 4 cases of BSE identified in the United States. The following information provides descriptions of these 4 cases:
- On December 23, 2003, the U.S. Department of Agriculture (USDA) announced a presumptive diagnosis of the first known case of BSE in the United States. It was in an adult Holstein cow from Washington State. This diagnosis was confirmed by an international reference laboratory in Weybridge, England, on December 25. Trace-back based on an ear-tag identification number and subsequent genetic testing confirmed that the BSE-infected cow was imported into the United States from Canada in August 2001. Because the animal was non-ambulatory (a "downer cow") at slaughter, brain tissue samples were taken by USDA's Animal and Plant Health Inspection Service as part of its targeted surveillance for BSE. However the animal's condition was attributed to complications from calving. After the animal was examined by a USDA Food Safety and Inspection Service (FSIS) veterinary medical officer both before and after slaughter, the carcass was released for use as food for human consumption. During slaughter, the tissues considered to be at high risk for the transmission of the BSE agent were removed. On December 24, 2003, FSIS recalled beef from cattle slaughtered in the same plant on the same day as the BSE positive cow. (see Bovine Spongiform Encephalopathy in a Dairy Cow - Washington State, 2003.)
- On June 24, 2005, the USDA announced receipt of final results from The Veterinary Laboratories Agency in Weybridge, England, confirming BSE in a cow that had conflicting test results in 2004. This cow was from Texas, died at approximately 12 years of age, and represented the first endemic case of BSE in the United States. (see Texas BSE Investigation, Final Epidemiology Report, August 2005

 (PDF – 83 KB))
(PDF – 83 KB))
- On March 15, 2006, the USDA announced the confirmation of BSE in a cow in Alabama. The case was identified in a non-ambulatory (downer) cow on a farm in Alabama. The animal was euthanized by a local veterinarian and buried on the farm. The age of the cow was estimated by examination of the dentition as 10-years-old. It had no ear tags or distinctive marks; the herd of origin could not be identified despite an intense investigation (see second featured item above and Alabama BSE Investigation, Final Epidemiology Report, May 2006

 (PDF – 104 KB)). In August 2008, several ARS investigators reported that a rare, genetic abnormality that may persist within the cattle population "is considered to have caused" BSE in this atypical (H-type) BSE animal from Alabama. (See Identification of a Heritable Polymorphism in Bovine PRNP Associated with Genetic Transmissible Spongiform Encephalopathy: Evidence of Heritable BSE
(PDF – 104 KB)). In August 2008, several ARS investigators reported that a rare, genetic abnormality that may persist within the cattle population "is considered to have caused" BSE in this atypical (H-type) BSE animal from Alabama. (See Identification of a Heritable Polymorphism in Bovine PRNP Associated with Genetic Transmissible Spongiform Encephalopathy: Evidence of Heritable BSE  . Also see BSE Case Associated with Prion Protein Gene Mutation
. Also see BSE Case Associated with Prion Protein Gene Mutation  .)
.)
- On April 24, 2012, the USDA confirmed a BSE case in a dairy cow in California. This cow was tested as part of the USDA targeted BSE surveillance at rendering facilities in the United States. The cow was 10 years and 7 months old and was classified as having the L-type BSE strain. For more information, see the USDA/APHIS notice
 , the USDA/APHIS Final Report
, the USDA/APHIS Final Report 
 (PDF – 179 KB) and the FDA Final Feed Investigation Summary.
(PDF – 179 KB) and the FDA Final Feed Investigation Summary. 
For more information about BSE in the United States, see: USDA, Animal Health, Bovine Spongiform Encephalopathy (BSE)  from the United States Department of Agriculture (USDA).
from the United States Department of Agriculture (USDA).
BSE Cases Identified in Canadian-born Cattle
As of March 2011, 19 BSE cases in Canadian-born cattle have been identified, 18 in Canada and 1 in the U.S. Of these 19 cases, 13 were known to have been born after the implementation of the 1997 Canadian feed ban  ; 12 of these 13 were born after March 1, 1999. (See Figure above: BSE Cases in North America, by Year and Country of Death, 1993-03/2011). This latter date is particularly relevant to the U.S. because since a USDA rule went into effect on November 19, 2007, Canadian cattle born on or after March 1, 1999 have been legally imported into this country for any use. One of the 19 Canadian-born BSE cases was reported in an animal that was most likely born before or possibly very shortly after implementation of the 1997 feed ban. Based on the known or most likely year of birth, an average of 1.4 cases of BSE occurred among the group of animals born each year in Canada from 1991 through 2004. The highest reported number of cases by birth year in a single year, 3 BSE cases, occurred in 2000, 2001 and 2002. The most recently reported case extends the period of BSE transmission in Canada through at least the latter half of 2004.
; 12 of these 13 were born after March 1, 1999. (See Figure above: BSE Cases in North America, by Year and Country of Death, 1993-03/2011). This latter date is particularly relevant to the U.S. because since a USDA rule went into effect on November 19, 2007, Canadian cattle born on or after March 1, 1999 have been legally imported into this country for any use. One of the 19 Canadian-born BSE cases was reported in an animal that was most likely born before or possibly very shortly after implementation of the 1997 feed ban. Based on the known or most likely year of birth, an average of 1.4 cases of BSE occurred among the group of animals born each year in Canada from 1991 through 2004. The highest reported number of cases by birth year in a single year, 3 BSE cases, occurred in 2000, 2001 and 2002. The most recently reported case extends the period of BSE transmission in Canada through at least the latter half of 2004.
Strains of BSE
There is increasing evidence that there are different strains of BSE: the typical BSE strain responsible for the outbreak in the United Kingdom and two atypical strains (H and L strains).
Typical BSE strain -- The BSE strain responsible for most of the BSE cases in Canada is the same classic or typical strain linked to the outbreak in the United Kingdom. It is known to be preventable through elimination of BSE contaminated feed and has been causally linked to vCJD in humans. This typical strain has not yet been identified in any U.S.-born cattle.
Atypical BSE strain -- In July 2007, the UK Spongiform Encephalopathy Advisory Committee (SEAC) suggested that atypical BSE may be a distinct strain of prion disease. Unlike typical BSE, cases of atypical BSE, according to SEAC, may have risen spontaneously (although transmission through feed or the environment cannot be ruled out). Recently reported French surveillance data support this theory that unlike typical BSE, atypical BSE appears to represent sporadic disease
All (3) of the U.S.-born BSE cases and two of the 19 Canadian-born BSE cases were 10 years of age or older and caused by atypical BSE strains. Of these 5 older North American cases, 3 were linked to an atypical BSE strain known as the H-type. The strain type for the other two older North American cases, a 13-year-old BSE-infected Canadian cow and a 10-year-old BSE-infected US cow, have been identified as the L-type.
For additional data on this topic: http://www.cdc.gov/eid/content/14/2/298.htm
Feed Bans
As of October 26, 2009, a regulation issued by FDA in April 2009 came into effect establishing an enhanced BSE-related feed ban in the United States. This enhanced ban will further harmonize BSE feed control measures in the U.S. with those in Canada (see below). In addition, FDA continues to enforce its important 1997 mammalian-to-ruminant feed ban through its BSE inspection and BSE feed testing programs.
As of July 12, 2007, an enhanced BSE-related feed ban  came into effect in Canada. CFIA
came into effect in Canada. CFIA  established this ban to more effectively prevent and quickly eliminate BSE from Canada. The enhanced ban prohibits most proteins, including potentially BSE infectious tissues known as “specified risk materials” (SRM) from all animal feeds, pet foods, and fertilizers, not just from cattle feed as required by the ban instituted in 1997. The 1997 feed ban in Canada was similar to the feed ban instituted in the United States that same year. As recently reported by CFIA, removing SRM from the entire animal feed system addresses risks associated with the potential contamination of cattle feed during production, distribution, storage, and use. Applying the same measure to pet food and fertilizer materials addresses the possible exposure of cattle and other susceptible animals to these products. CFIA expects that with this new ban, BSE should be eliminated from the Canadian cattle herd by about the year 2017.
established this ban to more effectively prevent and quickly eliminate BSE from Canada. The enhanced ban prohibits most proteins, including potentially BSE infectious tissues known as “specified risk materials” (SRM) from all animal feeds, pet foods, and fertilizers, not just from cattle feed as required by the ban instituted in 1997. The 1997 feed ban in Canada was similar to the feed ban instituted in the United States that same year. As recently reported by CFIA, removing SRM from the entire animal feed system addresses risks associated with the potential contamination of cattle feed during production, distribution, storage, and use. Applying the same measure to pet food and fertilizer materials addresses the possible exposure of cattle and other susceptible animals to these products. CFIA expects that with this new ban, BSE should be eliminated from the Canadian cattle herd by about the year 2017.
The Canadian-born cow confirmed to be infected with BSE in 2010 illustrates the difficulty in determining the effectiveness of previously instituted feed bans to prevent BSE transmissions. The initial feed bans established in both the United States and Canada were instituted in 1997. After an assessment by USDA and its Canadian counterparts, the Canadian feed ban was judged to be fully effectiveness as of March 1999. However, largely because of recognized limitations of this ban and the ban established in the United States, new, enhanced feed bans went into effect in Canada, July 12, 2007, and in the U.S., October 26, 2009. While USDA has confirmed no U.S.-born cattle as having a classic form of BSE, Canadian cattle born after March 1999 have been legally imported into the United States for any purpose since November 19, 2007.
BSE Prevalence
Based on World Organization of Animal Health (OIE) standards for BSE surveillance, the reported national prevalence rates of BSE in North American cattle, particularly in animals born in the United States, is very low, and therefore, difficult to measure accurately. In September 2007, the USDA published updated results of the two statistical models used by Harvard University investigators to estimate the prevalence of BSE in Canada. The results incorporated the 11 Canadian-born animals with BSE that had been reported at that time. A key advantage of these models is that they provide statistical confidence limits that measure some of the uncertainty associated with expected estimates. To view the results of the model, called BSurvE, that is most comparable to the observed surveillance data, see page 15 of the document titled "Response to peer review of risk assessment of Bovine Spongiform Encephalopathy (BSE) risks associated with the importation of certain commodities from BSE minimal risk regions (Canada)" 
 (PDF – 261 KB). This model estimated that the true prevalence of BSE in Canada has been 90% likely to be between 18-fold and 48-fold higher than the previously published best estimate of the prevalence of BSE in the United States (3.0 to 8.0 cases per million in Canada compared to a best estimate of 0.167 cases per million in the United States [reference #3]). The previously published best estimate of Canada’s BSE prevalence in 2006 using the BSurveE model was 23-fold higher than that of the United States and is the estimate of the BSE prevalence in Canada that continues to be used in the Harvard Risk Assessments’ "worst case" analyses when evaluating the risk of imported Canadian cattle causing BSE to spread among US animals
(PDF – 261 KB). This model estimated that the true prevalence of BSE in Canada has been 90% likely to be between 18-fold and 48-fold higher than the previously published best estimate of the prevalence of BSE in the United States (3.0 to 8.0 cases per million in Canada compared to a best estimate of 0.167 cases per million in the United States [reference #3]). The previously published best estimate of Canada’s BSE prevalence in 2006 using the BSurveE model was 23-fold higher than that of the United States and is the estimate of the BSE prevalence in Canada that continues to be used in the Harvard Risk Assessments’ "worst case" analyses when evaluating the risk of imported Canadian cattle causing BSE to spread among US animals
For more information on this topic:
- An Estimate of the Prevalence of BSE in the United States, July 20, 2006

 (PDF – 70 KB)
(PDF – 70 KB)
- APHIS, USDA, Assessment of Bovine Spongiform Encephalopathy risks associated with the importation of certain commodities from BSE minimal risk regions (Canada), Attachment 1, October 27, 2006

 (PDF – 279 KB)
(PDF – 279 KB)
- APHIS, USDA’s BSE Surveillance Efforts Factsheet, July 2006

 (PDF – 56 KB)
(PDF – 56 KB)
For regularly updated numbers of reported BSE cases worldwide, see the Office International Des Epizooties (OIE) BSE website  .
.
For information about BSE in Canada, see the Canada Food Inspection Agency (CFIA) website  .
.
 Topic Contents:
Topic Contents:
 See Also:
See Also: