Human Subject Regulations Decision Charts
Human Subject Regulations Decision Charts
September 24, 2004
The Office for Human Research Protections (OHRP) provides the following graphic aids as a guide for institutional review boards (IRBs), investigators, and others who decide if an activity is research involving human subjects that must be reviewed by an IRB under the requirements of the U.S. Department of Health and Human Services (HHS) regulations at 45 CFR part 46. OHRP welcomes comment on these decision charts. The charts address decisions on the following:
- whether an activity is research that must be reviewed by an IRB
- whether the review may be performed by expedited procedures, and
- whether informed consent or its documentation may be waived.
Considerations
The charts are intended to assist IRBs, institutions, and investigators in their decision-making process and should not be used as substitutes for consulting the regulations. OHRP cautions that the full text of applicable regulatory provisions should be considered in making final decisions.
These charts are necessarily generalizations and may not be specific enough for particular situations. Other guidance documents are available related to specific topics, at OHRP Policy Guidance by Topic. OHRP invites inquiries for additional information.
The charts do not address requirements that may be imposed by other organizations, such as the Food and Drug Administration, National Institutes of Health, other sponsors, or state or local governments.
Chart 1: Is an Activity Research Involving Human Subjects?
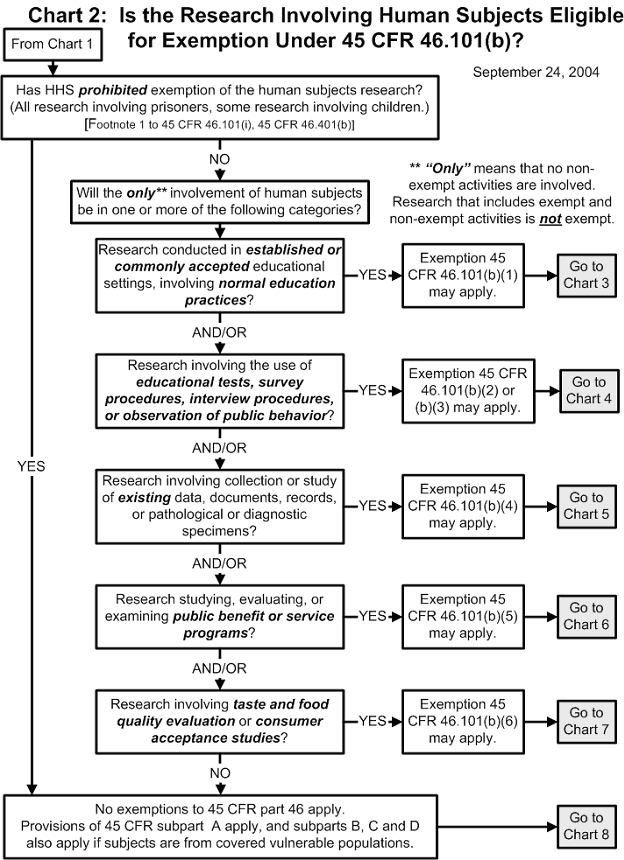
Chart 2: Is the Human Subjects Research Eligible for Exemption?
Chart 3: Does Exemption 45 CFR 46.101(b)(1) (for Educational Settings) Apply?
Chart 4: Does exemption 45 CFR 46.101(b)(2) or (b)(3) (for Tests, Surveys, Interviews, Public Behavior Observation) Apply?
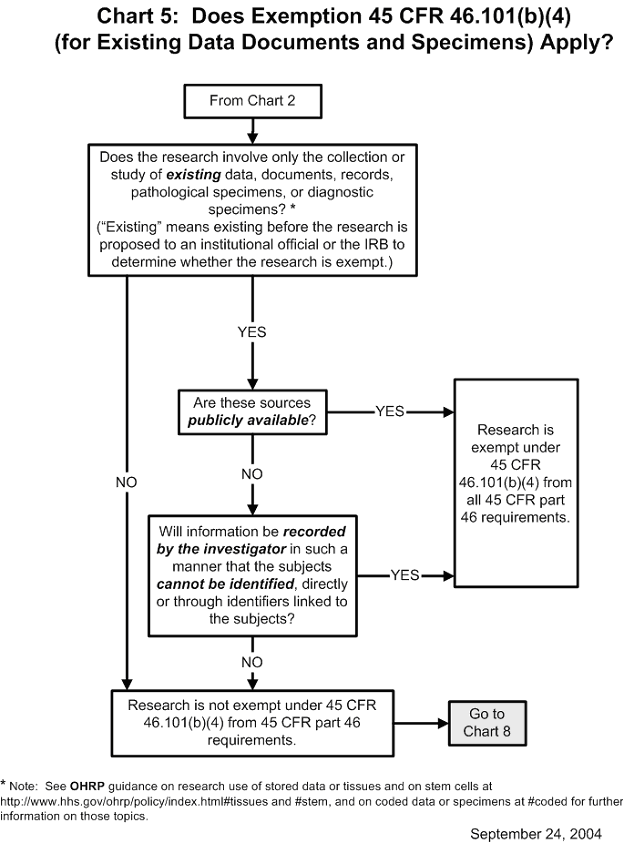
Chart 5: Does Exemption 45 CFR 46.101(b)(4) (for Existing Data, Documents, Records and Specimens) Apply?
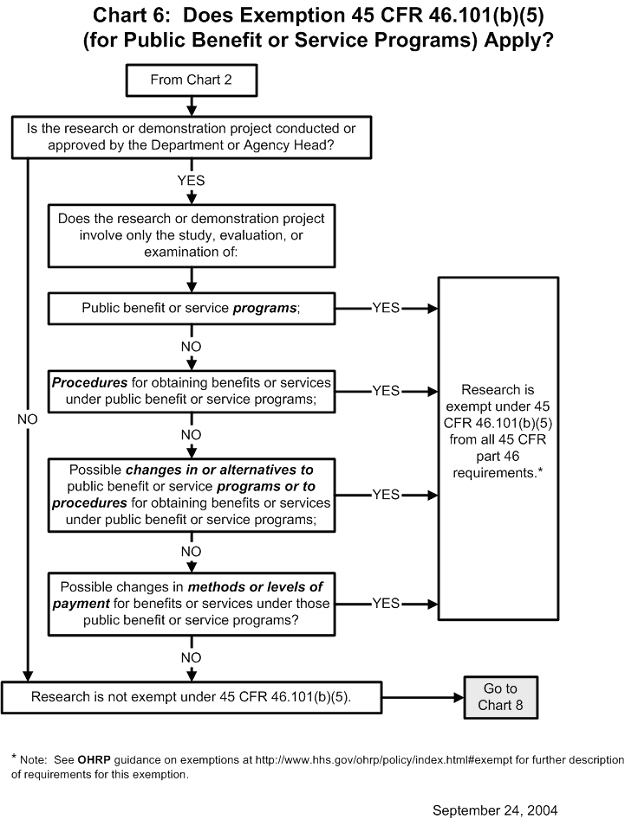
Chart 6: Does Exemption 45 CFR 46.101(b)(5) (for Public Benefit or Service Programs) Apply?
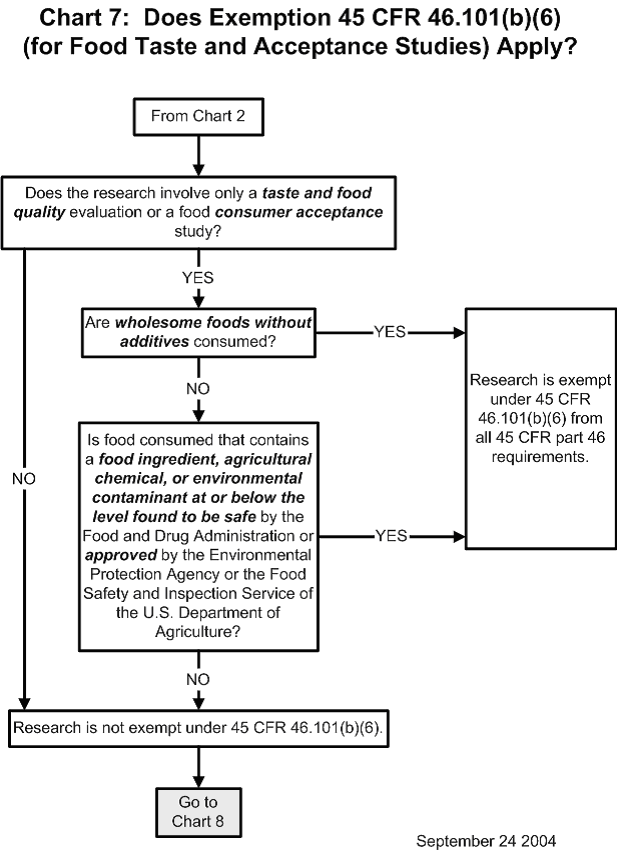
Chart 7: Does Exemption 45 CFR 46.101(b)(6) (for Food Taste and Acceptance Studies) Apply?
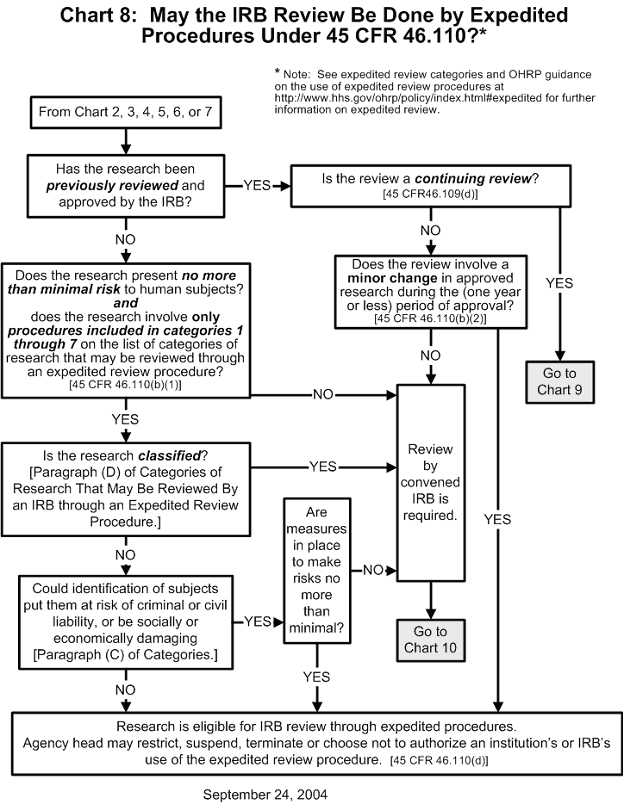
Chart 8: May the IRB Review Be Done by Expedited Procedures?
Chart 9: May the IRB Continuing Review Be Done by Expedited Procedures?
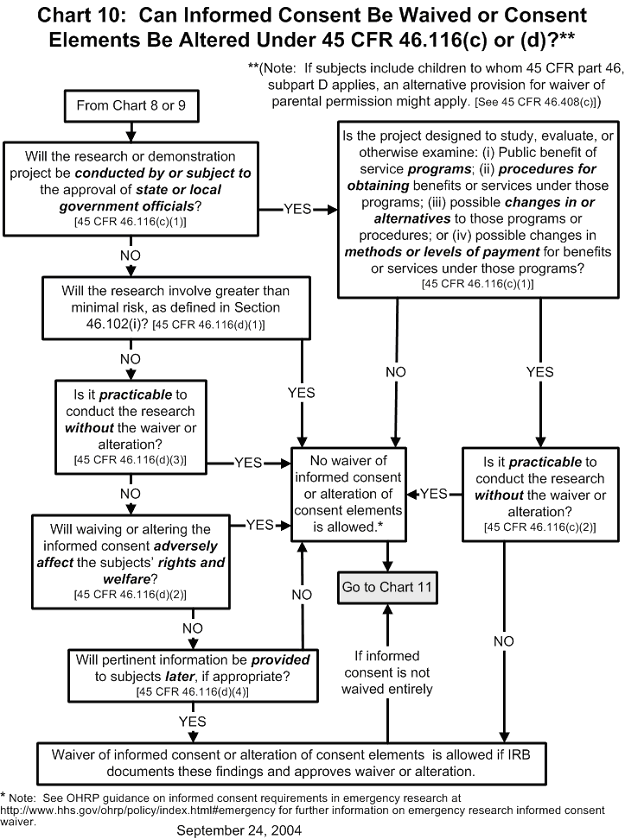
Chart 10: May Informed Consent Be Waived or Consent Elements Be Altered under 45 CFR 46.116(d)?
Chart 11: May Documentation of Informed Consent Be Waived Under 45 CFR 46.117(c)?
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |





