Tobacco Products
New Tobacco Product Review and Evaluation
How do I legally market my new tobacco product?
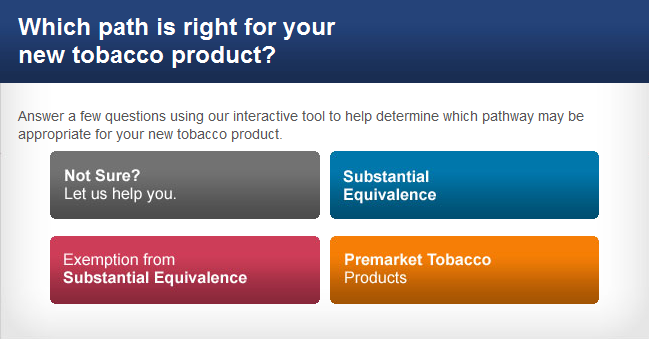
To legally market a new tobacco product in the United States, you must receive a written order from FDA permitting the marketing of your new tobacco product under one of three pathways. You can submit:
- A Substantial Equivalence Report
- An Exemption from Substantial Equivalence Request or
- A Premarket Tobacco Application
According to the Food, Drug & Cosmetic Act, a new tobacco product is any product that was not commercially marketed in the United States as of February 15, 2007. This includes tobacco products that were modified and marketed after February 15, 2007. (see Section 910(a)(1).
Click the image below to visit the page with our interactive tool to help you determine which path is right for your new tobacco product.
This web content highlights provisions of the Food, Drug and Cosmetic Act (FD&C Act). It is not intended to be comprehensive or reflect FDA's interpretation of the Act. For complete information, you must read the entire law. For your convenience, the section number of the FD&C Act is referenced and relevant sections are linked to throughout the web content.