For Consumers
Warning on Body Building Products Marketed as Containing Steroids or Steroid-Like Substances
|
For more videos, visit the FDA's YouTube Channel. |
Examples of products affected by FDA’s advisory.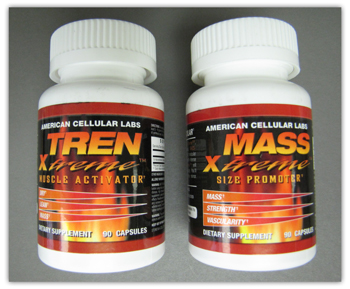 |
 Get Consumer Updates by E-mail
Get Consumer Updates by E-mail
On July 28, 2009, the U.S. Food and Drug Administration (FDA) issued a public health advisory warning consumers to stop using any body building products that are represented to contain steroids or steroid-like substances. Many of these products are marketed as dietary supplements.
This advisory was issued along with a warning letter sent to American Cellular Laboratories Inc. for marketing and distributing body building products containing synthetic steroid substances. Although these products are marketed as dietary supplements, they are NOT dietary supplements, but instead are unapproved and misbranded drugs.
Q. What types of products are affected by this public health advisory?
A. FDA is warning consumers about products that are being marketed for body building and that claim to contain steroids or steroid-like substances. These products are sold online and in retail stores and are promoted as hormone products and/or as alternatives to anabolic steroids for increasing muscle mass and strength. Many of these products are labeled as dietary supplements and make claims about the ability of the active ingredients to enhance or diminish androgen, estrogen, or progestin-like effects in the body. Consumers should be aware that these products are potentially harmful and that FDA has not approved them nor reviewed their safety before marketing.
Q. What are some examples of these types of products?
A. These body building products are often marketed as being anabolic (promoting muscle building) and/or being similar to anabolic steroids (such as testosterone). The products included in the warning letter to American Cellular Laboratories Inc. provide a few examples of the body building products about which FDA has safety concerns. The product names and ingredients listed in the warning letter are:
- TREN-Xtreme: 19-Norandrosta-4,9-diene-3,17 dione, marketed as “similar to Trenbolone”
- MASS Xtreme: 17α-methyl-etioallocholan-2-ene-17b-ol, marketed as “similar to Methyl Testosterone”
- ESTRO Xtreme: 4-hydroxyandrostenedione (4-OHA)
- AH-89-Xtreme: 5α-androstano[3,2-c]pyrazole-3-one-17β-ol-THP-ether, marketed as “similar to Stanozolol”
- HMG Xtreme: 2α,3α-epithio-17α-methyl-17β-hydroxy-5α-etioallocholane
- MMA-3 Xtreme: Androsta-1,4-dien-3,17-dione, marketed as “similar to Boldenone (Equipoise)”
- VNS-9 Xtreme: 17α-methyl-4-chloro-androsta-1,4-diene-3β,17β-diol, marketed as “similar to Turinabol”
- TT-40-Xtreme: 1-androsterone, marketed as “very similar to 1-Testosterone” and “converts to 1-Testosterone”
Q. What are the health risks of these types of products?
A. Adverse event reports received by FDA for body building products that are labeled to contain steroids or steroid alternatives involve men (ages 22-55) and include cases of serious liver injury, stroke, kidney failure and pulmonary embolism (blockage of an artery in the lung). Acute liver injury is known to be a possible harmful effect of using anabolic steroid-containing products. In addition, anabolic steroids may cause other serious long-term adverse health consequences in men, women, and children. These include shrinkage of the testes and male infertility, masculinization of women, breast enlargement in males, short stature in children, adverse effects on blood lipid levels, and increased risk of heart attack and stroke.
Q. Why does FDA say these products are illegally marketed?
A. These products are NOT dietary supplements because they contain synthetic steroid or steroid-like active ingredients. These products are unapproved new drugs because they are not generally recognized as safe and effective. In fact, they are potentially harmful. In addition, the products are misbranded because the labeling is misleading and does not provide adequate directions for use.
Q. What action is FDA taking?
A. FDA has issued a public health advisory to highlight the risks of products that are marketed for body building and that contain or claim to contain steroids or steroid-like substances. The agency has executed a search warrant and issued a warning letter to American Cellular Laboratories Inc., which markets a number of these products, because the products are unapproved new drugs and are misbranded. FDA is gathering and reviewing additional data about other products that are marketed for body building and that claim to contain steroids or steroid-like substances.
Q. What should consumers do if they have been using these products?
A. Due to the potential serious health risks, FDA recommends that consumers immediately stop using these products. Consumers should also consult their health care professional if they are experiencing symptoms possibly associated with these products, particularly nausea, weakness or fatigue, fever, abdominal pain, chest pain, shortness of breath, yellowing of the skin or whites of the eyes, or brown/discolored urine. FDA also recommends that consumers talk with their health care professional about body building supplements they are taking, particularly if they are uncertain about the product’s ingredients.
These products are often promoted to athletes to improve sports performance and to aid in recovery from training and sporting events. FDA cautions that athletes taking these products may test positive for performance-enhancing drugs.
Q. How should consumers report problems to FDA?
A. Report serious side effects or product quality problems with the use of these products to FDA's MedWatch Adverse Event Reporting program online, by regular mail, fax, or phone.
- Online
- Regular Mail: Use FDA postage paid form 3500 and mail to MedWatch, 5600 Fishers Lane, Rockville, MD 20852-9787
- Fax: 800-FDA-0178
- Phone: 800-FDA-1088
This article appears on FDA's Consumer Updates page, which features the latest on all FDA-regulated products.
Date Posted: July 28, 2009








