For Consumers
Emerging Technology Embraces the Future
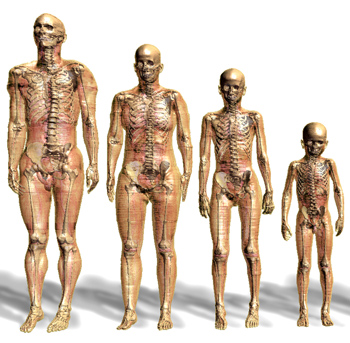 |
The original Virtual family starts with the second figure from the left, the 34-year-old adult male. Next to him are the original 26-year-old female and two children, an 11-year-old girl and 6-year-old boy. Since the creation of these four original figures, researchers have developed an extended family of anatomically correct computer models with different body types and ages. |
The Impact on You Regulatory science is the science of developing new tools, standards and approaches to assess the safety, effectiveness, quality and performance of FDA-regulated products. Explore how regulatory science will affect your daily life by: |
 Get Consumer Updates by E-mail
Get Consumer Updates by E-mail
 Share copies of this article (934 KB)
Share copies of this article (934 KB)
Emerging technology may be the rock star of regulatory science.
In this science that is the foundation of regulatory decisions by the Food and Drug Administration (FDA), emerging technology has the flash and dazzle. Scientists at FDA’s Center for Devices and Radiological Health (CDRH) are using the kind of technology you’d expect to see in a video game or sci-fi movie.
“We help support innovation and the development of new technology. And to do that we need to advance our own technology that will help us evaluate and enhance our understanding of these new medical devices,” says Michelle McMurry-Heath, M.D., Ph.D., associate director for science at CDRH.
Here are some of the new technologies being developed and used at CDRH:
The Virtual Family
The computer-generated dad, mom and two kids make up a virtual model family. The adult male and female and the children are based on body scans of healthy volunteers, including more than 80 types of tissue, including tissue from the bone, brain and heart.
These anatomically correct models will be used to investigate how medical devices interact with the body. They’ve already been used to test the body temperatures around implanted devices when people are exposed to radio frequencies—such as those used in cell phones and Bluetooth technologies—and microwave radiation.
One of the goals is to allow developers to test early versions of their new devices on these computer models instead of on real people. This could reduce costs and speed the development of new products.
The Virtual Family is also being used to predict the best way to defibrillate children in the midst of a cardiac crisis. (Defibrillation uses an electric current to shock a heart with a severely abnormal, and life-threatening, rhythm back into a normal rhythm.) Researchers are identifying the best placement of defibrillator leads and the right amount of electrical energy.
There will eventually be a library of computer models like these, says McMurry-Heath. Clinicians will be able to call them up and input information specific to their patients to determine which device might work better for them.
Phantom Models
Because FDA is concerned about the amount of radiation patients are exposed to from diagnostic tests, CDRH scientists are using physical models they call “phantoms” to understand how different imaging technologies work without having to subject an actual person to radiation.
These aren’t phantoms in the way you might imagine. “They’re more like crash-test dummies,” says Steven Pollack, Ph.D., director of FDA’s Office of Science and Engineering Laboratories.
These phantoms allow researchers to test different imaging methods—such as X-ray and ultrasound—on the same subject, Pollack says. This enables scientists to weigh the risk versus the benefits of different technologies without exposing a patient to potential harm.
Researchers insert materials into a phantom model that mimic tumors and then take an image to see how clear and precise the picture will be at various levels of radiation exposure.
Robotics
The use of robotics in medical applications is rapidly growing in scope and complexity.
For example, FDA researchers are collaborating with the Defense Advance Research Projects Agency (DARPA) to develop artificial limbs that are controlled by the patient’s own nervous system. DARPA is an agency within the Department of Defense that has funded the development of many major technologies, including computer networking. CDRH and DARPA scientists are studying the next generation of prosthetic limbs that could be controlled by a chip in the patient’s brain, Pollack says.
The questions they’re wrestling with include: How long does the implant chip reliably send signals and what can be done—either by using novel materials or by tricking the body into ignoring it (not attacking it as a foreign presence)—to increase that time?
Smart Devices
The development of “smart” medical devices is on the rise. FDA has cleared some mobile medical applications used by health care professionals, such as a smartphone-based ultrasound and an application for iPhones and iPads that allows doctors to view medical images such as X-rays and sonograms. (Sonograms are images captured through the use of sound waves.)
Pollack explained that FDA researchers are examining how the user’s eye scans information received this way to gain a better understanding of how it is viewed and interpreted. The ultimate goal, he says, is to determine if there are improvements needed to the device or software.
Maintaining Radio Silence
Called an anechoic chamber, this large, padded room at FDA keeps out radio signals from the surrounding environment. (Radio signals don’t just come from radios, but also from such communication staples as cell phones and wireless networks.) Within its walls, researchers expose medical devices to electromagnetic waves, explains Joel Myklebust, P.E., Ph.D., deputy director at OSEL. The goal is to see how a device—such as a pacemaker—will react to an electromagnetic source (such as an X-ray or certain electronic devices).
What would happen, for example, if you have a pacemaker and need an MRI (magnetic resonance imaging) examination? What levels are safe? In a hospital, how is a device affected if everyone in the vicinity has cell phones or computer laptops?
The chamber is also used to test the impact of radiowaves, such as those used in the identification tags used to identify and track products (in stores and warehouses) and people (employee IDs, farecards, etc.). Myklebust notes that some of these radio-frequency emitters have been found to temporarily slow the rate of pacemakers and affect other implanted cardiac devices.
Information from this testing is shared with medical device manufacturers and makers of identification equipment.
These are just a sampling of the technologies being developed across FDA as part of the agency’s commitment to regulatory science. “We’re always scanning the horizon to see what’s going on in laboratories, what technologies may be coming so that we can stay ahead of the curve,” says Pollack.
This article appears on FDA's Consumer Updates page, which features the latest on all FDA-regulated products.
September 17, 2012








