For Consumers
Building a Stronger Defense Against Bioterrorism
Printer-friendly PDF (734 KB)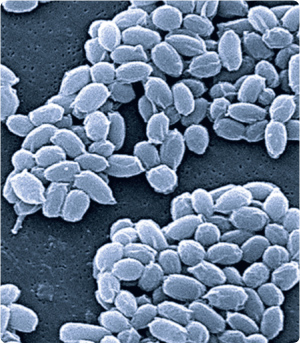 | |
An electron micrograph of spores from the Sterne strain of Bacillus anthracis, the bacterium that causes anthrax. |
 Get Consumer Updates by E-mail
Get Consumer Updates by E-mail
On this page
- High-Priority Threat Agents
- Protecting the Food Supply
- Effective Medical Products
- Emergency Use Authorizations (EUAs) of Medical Products
- The "Animal Rule"
- Guarding the Blood Supply
Spurred by the horrendous events of September 11, 2001, the Food and Drug Administration has worked to prevent and mitigate the effects of bioterrorism, and to be prepared if a bioterrorist attack should ever occur.
"We've made very important contributions toward protecting the nation against its enemies," says Boris Lushniak, MD, MPH, FDA's Assistant Commissioner for Counterterrorism Policy.
Bioterrorism refers to a deliberate release of viruses, bacteria, fungi or toxins from living organisms to cause illness or death in people, animals, or plants. Harmful agents can be spread through the air, water, or in our food.
In the event that a biological agent is released in the United States, FDA would work closely with federal, state, and local authorities to investigate the problem and get contaminated products off the market quickly.
"FDA's role is very challenging," says Lushniak, who leads FDA's Office of Counterterrorism and Emerging Threats (OCET). "We need to balance public health needs, regulatory requirements, and science, as we assist with defense of the nation."
High-Priority Threat Agents
"In keeping with national policies," Lushniak says, "FDA's counter-terrorism efforts focus largely on what the U.S. Department of Health and Human Services (HHS) has identified as the highest priority threat agents and diseases for medical countermeasure development and acquisition."
Bioterrorism Legislation Laws passed as a result of the 2001 attacks have allowed FDA to enact bold, wide-ranging initiatives that help anchor the country"s defense strategy. |
These agents and diseases are (in alphabetical order):
- anthrax, including multi-drug resistant anthrax
- Burkholderia bacteria
- Clostridium botulinum toxins
- plague
- radiological/nuclear agents
- smallpox
- typhus
- tularemia
- various viral hemorrhagic fevers
- volatile nerve agents
Protecting the Food Supply
FDA efforts to protect the food supply include:
Developing new regulations. The Bioterrorism Act of 2002 led FDA to develop major regulations requiring registration of food facilities, prior notification of imported food shipments, and establishment and maintenance of records. Lushniak says, "It has also empowered the agency to detain any food for up to 30 days when there is credible evidence that the food poses a serious threat to humans or animals."
Increasing surveillance. New regulations have allowed FDA to increase surveillance of domestic and imported food, inspections of domestic food facilities, and sampling and lab analysis of foods produced here and abroad.
 |
U.S. Food and Drug Administration FDA inspectors take samples of crushed red pepper from a shipment in a food warehouse at Baltimore Harbor. |
Developing and testing technologies. Working with the food industry and other federal agencies, FDA has funded or conducted more than 100 research projects aimed at developing tests and sampling methods for detecting adulterated food.
Working to reduce threats and vulnerabilities. FDA issued voluntary guidance in 2003 to help the food industry minimize the risk that its products will be subject to terrorism. FDA has also collaborated with other federal agencies to develop CARVER + Shock, an assessment methodology that allows the food and agriculture industries to identify vulnerabilities.
CARVER + Shock is used by the Strategic Partnership Program Agroterrorism (SPPA) Initiative, a collaboration between federal and state agencies, trade associations and the food and agriculture industries. FDA has released a software version of the methodology that allows industry to conduct customized vulnerability assessments for its food production facilities.
Effective Medical Products
FDA is responsible for ensuring the safety and effectiveness of medical products for diagnosing, treating, and preventing bioterrorism-linked illness.
FDA has previously licensed vaccines for anthrax and smallpox, as well as antitoxin to treat victims of Botulinum poisoning. Additionally, there are products in development that include new vaccines or treatments against anthrax, smallpox, botulism, plague, tularemia, and Ebola, among other organisms.
Part of the agency's role in a biological emergency would be to make promising treatments—including drugs, vaccines, blood products and blood derivatives, gene therapies, and cells and tissues for transplantation—readily available.
FDA's ability to get as many safe and effective medical products as possible—including promising treatments—out to the public during a biological emergency has been boosted by two recently enacted measures.
Emergency Use Authorizations (EUAs) of Medical Products.
Project BioShield, signed into law in July 2004, provides FDA authority to issue EUAs during emergencies involving a heightened risk of attack on the public or U.S. military forces, or when there is significant potential of an event that would affect national security.
An EUA authorizes the use of unapproved medical products—and unapproved uses of approved medical products—to diagnose, treat, or prevent serious or life-threatening disease or conditions caused by chemical, biological, radiological, and nuclear agents.
An EUA can be issued once the Secretary of HHS declares a qualifying emergency, and after FDA's commissioner determines that certain criteria are met. The decision on issuing an EUA would be made in consultation with the directors of the Centers for Disease Control and Prevention and the National Institutes of Health (or their delegates), where practicable.
This counterterrorism measure, officially known as "Approval of Biological Products/New Drugs when Human Efficacy Studies are not Ethical or Feasible," was put in effect in 2002.
It allows FDA to approve biologics and drugs for chemical, biological, radiological, or nuclear agents based on evidence of effectiveness from animal studies if the product has been shown to be safe in humans.
"Data from animal-studies must be sufficient to establish effectiveness in humans," says Lushniak. Such effectiveness can be established when
- the biological agent's mechanism of toxicity is well understood
- endpoints in the animal trials are clearly related to benefit in humans
- the product's effect is demonstrated in a species expected to react similarly to humans
- data allow selection of an effective human dose
To date, FDA has approved two products under the Animal Rule. Pyridostigmine bromide was approved in February 2003 for combat use by U.S. military personnel to increase survival after exposure to Soman "nerve gas" poisoning. In December 2006, FDA approved Cyanokit for the treatment of known or suspected cyanide poisoning.
Guarding the Blood Supply
FDA is also tasked with helping keep the blood supply safe.
A bioterror-induced outbreak of disease may lead to mass vaccinations requiring use of live viruses. Since people who are immunized cannot donate blood for a period of time because they may transmit the vaccine virus, guidance has been issued on reducing the risk of transmitting diseases through blood donated by people who have been either exposed to a live vaccine or to a biological agent.
Date Posted: August 29, 2007








