| * |
|
Ceramic Phase Equilibrium Data
Summary:
Description:Phase equilibrium data are used throughout the ceramics industry to understand and control the complex phenomena that underlie the production and performance of advanced materials. Phase diagrams serve as maps of the equilibrium chemical and structural behaviors exhibited by materials and provide critical starting information for the rational design of materials processing schemes, quality assurance efforts, and optimization of the physical and chemical properties of advanced materials. The Phase Equilibria Diagrams series (NIST Standard Reference Database 31) provides critical
Impact and Customers:
Major Accomplishments:Recent activities in the Data Center have concentrated on using a newly redesigned Content Management System (CMS) to produce updated versions of the CD-ROM database.
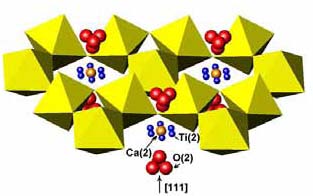
Version 3.3, Phase Equilibrium Diagrams Database (SRD 31) The latest version of the database, CDROM v. 3.3, was released in June 2010. This update added 900 figures with approximately 1400 new phase diagrams and provides experimental and calculated data for an unprecedented range of non-organic material-types pertinent to a wide variety of applications. The chemical systems include phosphates (batteries, laser and other optoelectronic materials), chalcogenides (semiconducting sulfides, selenides and tellurides for thermoelectrics, optoelectronics, photovoltaics), numerous pnictide systems (nitrides, phosphides, arsenides, and antimonides for bandgap-engineered optical materials, electron-transport devices, sensors, detectors, photovoltaics, thermoelectrics), and halides (nuclear applications, scintillation detectors) in addition to numerous oxide and metal+oxide systems (electrode processing, catalysis, electroceramics, magneto-resistors, thermistors, capacitors, nuclear fuel and nuclear waste, ionic conductors, fuel-cell electrolytes). CDROM v. 3.3 also includes all the information previously printed in the 21 hard-copy volumes of the series, with a total of more than 23,400 phase diagrams, and offers additional features such as export capability. A free demonstration CD is available upon request or can be downloaded from the website http://www.nist.gov/ts/msd/srd/nist31.cfm. SRD 31 provides evaluated graphical representations of phase equilibrium data in addition to written commentaries. Recent experimental phase equilibrium research has included studies of dielectric ceramic systems of interest to components for communications systems. Studies of the CaO: TiO2:Nb2O5 system confirmed the formation of six ternary phases: pyrochlore (A2B2O6O´), and five members of the (110) perovskite-slab series Ca (Ti,Nb)O, with n = 4.5, 5, 6, 7, nn 3n+2 and 8. Relations in the quasibinary Ca2Nb2O7 CaTiO3 system were determined in detail. The practically important ceramic CaTiO3 forms solid solutions with Ca2Nb2O7 as well as CaNb2O6, resulting in a triangular single-phase perovskite region. Structural refinement of the pyrochlore CaTiNbO using single 1.461.381.117 crystal X-ray diffraction data were carried out, and indicated that Ti mixes on the A-type Ca sites as well as on the smaller octahedral B-type sites. Ca occupies the ideal 16d position, but Ti is displaced 0.7 Å to partially occupy a ring of six 96g sites, thereby reducing its coordination number from eight to five. The O´ oxygens in both pyrochlores were displaced 0.48 Å from the ideal 8b position to a tetrahedral cluster of 32e sites. Local structure (displacive disorder) in the Ca-Ti-(Nb,Ta)-O pyrochlore; possible sites for displaced atoms denoted by rings of blue (Ti) and clusters of red (O) spheres
The Ca-Ti-(Nb,Ta)-O pyrochlores exhibited dielectric relaxation similar to that observed for a number of Bi-containing pyrochlores, which also exhibit displacively disordered crystal structures. Observation of dielectric relaxation in the CaTi-(Nb,Ta)-O pyrochlores suggests that it arises from the displacive disorder, as opposed to the presence of polarizable lone-pair cations such as Bi3+, as has been commonly assumed. Selected Publications
|
 Start Date:October 27, 2008End Date:ongoingLead Organizational Unit:mmlStaff:Benjamin Burton Associated Products:Project Summary (PDF) Contact
Terrell Vanderah |
 written commentaries, evaluated graphical representations, bibliographic data, and analytical capabilities. The published portion of the database now includes over 21,000 phase diagrams contained in 21 books and a CD-ROM. Activities in the Ceramics Division Data Center include continuous addition of new database content (about 1000 new entries per year), dissemination of the data in printed and digital formats, and development of scientific software to facilitate access to and use of the data.
written commentaries, evaluated graphical representations, bibliographic data, and analytical capabilities. The published portion of the database now includes over 21,000 phase diagrams contained in 21 books and a CD-ROM. Activities in the Ceramics Division Data Center include continuous addition of new database content (about 1000 new entries per year), dissemination of the data in printed and digital formats, and development of scientific software to facilitate access to and use of the data.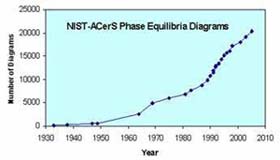 Advanced ceramics are critical enabling materials for a wide spectrum of devices and systems for electronic, communications, energy, medical, and chemical purification applications.
Advanced ceramics are critical enabling materials for a wide spectrum of devices and systems for electronic, communications, energy, medical, and chemical purification applications. Since 1933, NIST scientists have collaborated with the American Ceramic Society (ACerS) to meet the need for reliable phase diagram data by jointly publishing a series of critically evaluated collections of phase equilibrium diagrams.
Since 1933, NIST scientists have collaborated with the American Ceramic Society (ACerS) to meet the need for reliable phase diagram data by jointly publishing a series of critically evaluated collections of phase equilibrium diagrams.