| * |
|
International Comparison of Lu-177
Summary:There has been increasing interest during the past 10 years in the use of 177Lu for radionuclide-based radiotherapy for certain types of cancers. Accurate administrations of drugs using this radionuclide require accurate standards against which instrumentation used in the clinics and radiopharmacies can be calibrated. Several new 177Lu-based radiotherapy drugs are being investigated worldwide, which will cause an even greater need for such standards.
Description:
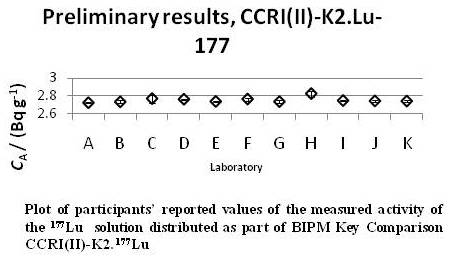
The only previous comparison of 177Lu that has been carried out was a bilateral comparison (BIPM-R(II)-K1.177Lu) conducted between the NIST and the Physikalisch-Technische Bundesanstalt (PTB) in 2000. In that case, both laboratories were able to submit ampoules to the International Reference System (SIR) and report activity values based on liquid scintillation counting using the Centro de Investigaciones Energéticas, Medioambientales y Tecnológicas (CIEMAT)/NIST efficiency tracing method. The results indicated a difference of about 1.4 % in the SIR equivalent activity. The short half-life of the 177Lu did not allow for follow-up studies to be performed. Since that time, several more National Metrology Institutes (NMIs) have standardized this radionuclide. In order to establish a link between primary standards of b-emitters in the NMIs and the SIR, as well as to provide a means for laboratories to substantiate Calibration and Measurement Capabilities (CMC) claims for β-γ nuclides, a CCRI(II) Key Comparison of 177Lu was carried out in the Spring of 2009. A total of 12 laboratories took part in the comparison, giving a total of 11 values to go into the calculation of the Key Comparison value. A plot of the reported values from each laboratory anonymized and multiplied by a normalization factor so as to not disclose the actual reported values. Although none of the values can strictly be considered an outlier, tests performed on the data indicate that there are inconsistencies in the data set. Analysis is underway to determine the best method to calculate the Key Comparison Reference Value (KCRV) while maintaining the integrity of the data set. The Draft A Report, including the calculation of the KCRV and Degrees of Equivalence, should be completed by early 2011. |
Lead Organizational Unit:pml |
 Lutetium-177 decays with three primary branches (E
Lutetium-177 decays with three primary branches (E