Radiation: Non-Ionizing and Ionizing
Ionizing & Non-Ionizing Radiation
Radiation has a wide range of energies that form the electromagnetic spectrum (illustrated below). The spectrum has two major divisions:
Radiation that has enough energy to move around atoms in a molecule or cause them to vibrate, but not enough to remove electrons, is referred to as "non-ionizing radiation." Examples of this kind of radiation include visible light and microwaves.
Radiation that falls within the "ionizing radiation" range has enough energy to remove tightly bound electrons from atoms, thus creating ions. This is the type of radiation that people usually think of as 'radiation.' We take advantage of its properties to generate electric power, to kill cancer cells, and in many manufacturing processes.
The energy of the radiation shown on the spectrum below increases from left to right as the frequency rises.
| Types of Radiation in the Electromagnetic Spectrum | |
|---|---|
  |
|
| Type of Radiation | |
| Effects | |
Source |
|
Nonionizing Radiation
We take advantage of the properties of non-ionizing radiation for common tasks:
- microwave radiation telecommunications and heating food
- infrared radiation infrared lamps to keep food warm in restaurants
- radio waves broadcasting
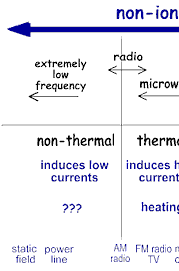
Non-ionizing radiation ranges from extremely low frequency radiation, shown on the far left through the audible, microwave, and visible portions of the spectrum into the ultraviolet range.
Extremely low-frequency radiation has very long wave lengths (on the order of a million meters or more) and frequencies in the range of 100 Hertz (cycles per second) or less. Radio frequencies have wave lengths of between one and 100 meters and frequencies in the range of one million to 100 million Hertz. Microwaves that we use to heat food have wavelengths that are about one hundredth of a meter and have frequencies of about 2.5 billion Hertz.
Ionizing Radiation
Higher frequency ultraviolet radiation begins to have enough energy to break chemical bonds. X-ray and gamma ray radiation, which are at the upper end of magnetic radiation, have very high frequencies (in the range of 100 billion billion Hertz) and very short wavelengths of about 1 picometer (1 trillionth of a meter). Radiation in this range has extremely high energy. It has enough energy to strip off electrons or, in the case of very high-energy radiation, break up the nucleus of atoms.
Ionization is the process in which a charged portion of a molecule (usually an electron) is given enough energy to break away from the atom. This process results in the formation of two charged particles or ions: the molecule with a net positive charge and the free electron with a negative charge.
Each ionization releases approximately 33 electron volts (eV) of energy. Material surrounding the atom absorbs the energy. Compared to other types of radiation that may be absorbed, ionizing radiation deposits a large amount of energy into a small area. In fact, the 33 eV from one ionization is more than enough energy to disrupt the chemical bond between two carbon atoms. All ionizing radiation is capable, directly or indirectly, of removing electrons from most molecules.
There are three main kinds of ionizing radiation:- alpha particles, which include two protons and two neutrons
- beta particles, which are essentially high-speed electrons
- gamma rays and x-rays, which are pure energy (photons).
![[logo] US EPA](https://cybercemetery.unt.edu/archive/oilspill/20120917002144im_/http://www.epa.gov/epafiles/images/logo_epaseal.gif)