- Stratospheric Ozone Layer Recovery Questions
- What is the Stratospheric Ozone Layer?
- Why is it important?
- What do we know about ozone depletion and recovery?
- What don't we know?
- What is NOAA's role in guiding the recovery of the ozone layer?
- What will we need to know in the future?
- What are the benefits for society of NOAA's activities?
- Programmatic Information
- Theme Presentations
Stratospheric Ozone Layer Depletion and Recovery
NOAA's role as a steward of the atmospheric environment has enabled it to play a central role in enhancing our understanding of the ozone layer and ozone layer depletion, and in gauging the effectiveness of measures taken to restore the ozone layer to its original strength. NOAA is charged to track the amount of ozone in the stratosphere (referred to here as the "thickness" of the stratospheric ozone layer, and the atmospheric burden of ozone-depleting compounds and their alternatives. NOAA additionally provides fundamental studies of the atmosphere and atmospheric processes to further our understanding of stratospheric ozone depletion and of the potential for recovery the ozone layer.
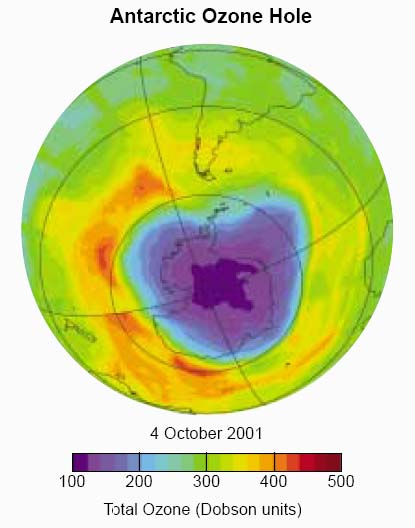
Antarctic "ozone hole". Total ozone values are shown for high southern latitudes as measured by a satellite instrument. The dark regions over the Antarctic continent show the severe ozone depletion now found in every spring. Minimum values of total ozone inside the ozone hole are close to 100 Dobson units (DU) compared with normal springtime values of about 300 DU [...]. In late spring or early summer (November- December) the ozone hole disappears as ozone-depleted air is displaced and diluted by ozone-rich air from outside the ozone hole.
What is the Stratospheric Ozone Layer and Ozone Layer Depletion?
Most ozone is found in a layer more than 10 kilometers (6 miles) above the Earth. This stratospheric ozone layer prevents the Sun's harmful, high-energy radiation from reaching Earth's surface.
Ozone in the stratosphere is constantly being created and destroyed by the action of light and photochemistry. The thickness of the ozone layer depends upon the balance of many different processes. The accumulation of chlorofluorocarbons and other ozone-depleting gases in the atmosphere as a result of human activities have altered this balance so that the ozone layer has become depleted. The depletion has been dramatic over certain regions of the globe since the 1980s, such as above Antarctica during September- October, but is less severe in other regions.
Why is it important?
The ozone layer acts as a shield for the planet that prevents dangerous radiation from reaching the biosphere, where humans, plants, and animals reside. The small amount of this high energy, ultraviolet radiation that normally reaches Earth's surface is responsible for sunburn, skin cancer, cataracts, and damage to genetic material. The depletion of ozone in the ozone layer results in increased amounts of this damaging radiation reaching Earth's surface and an increased occurrence of these problems in humans and other living beings.
- NOAA Data
- Observational Ozone Data
- Total Column Ozone
- Ozone Sonde Data
- Upper Troposphere and Lower Stratosphere Water Vapor
- Stratospheric Aerosol Data
- Halocarbon Data
- Halocarbons and other Atmospheric Trace Species (HATS) Archive
- Solar and Thermal Atmospheric Radiation (STAR)
- Ozone Assessment Summary
- Stratospheric Ozone Background Information
- 20 Questions and Answers About Ozone
What do we know about ozone depletion and recovery?
The stratospheric ozone layer has become substantially depleted throughout much of the globe since the 1980s because of enhanced human production and use of ozone- depleting chemicals, such as chlorofluorocarbons, halons, and others, during the 20th century. UV radiation increases in areas where the ozone layer has thinned. Ozone-depleting chemicals include those that contain chlorine or bromine and that are not easily removed from the atmosphere by chemical degradation or dissolution in clouds and rain. Atmospheric observations of these chemicals during the latter part of the 20th century showed dramatic increases that could be directly traced to the amounts produced by humans. By 1980, the amounts of chlorine and bromine from these chemicals soon far surpassed the smaller amounts of atmospheric chlorine and bromine arising from natural processes. The abnormally high quantities of atmospheric chlorine and bromine began altering the balance of ozone in the stratosphere so as to dramatically thin the ozone layer. Since the 1980s the most severe ozone layer depletion has been regularly observed over Antarctica during spring, when ozone levels drop by over 95% and UV radiation reaching Earth's surface increases substantially. Less intense depletion of ozone occurs above the Arctic and in mid-latitudes of both hemispheres.
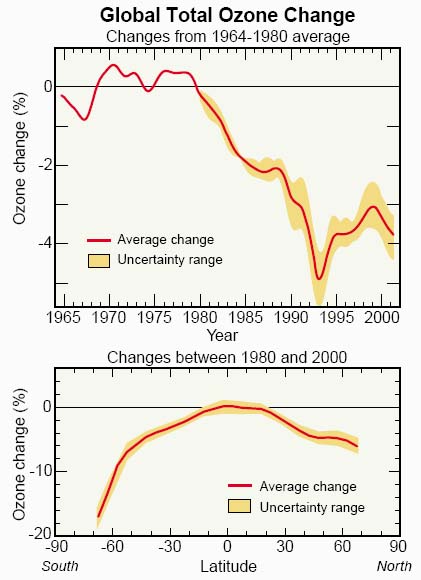
Global total ozone changes. Global total ozone values decreased by an average of a few percent in the last two decades, as measured by satellite instruments. In the top panel, global ozone changes are compared with average global ozone found in the period of 1964 to 1980. Between 1980 and 2000, the largest decreases occurred following the volcanic eruption of Mt. Pinatubo in 1991. In the 1997 to 2001 period global ozone was reduced by about 3% from the 1964- 1980 average.
In the bottom panel, ozone changes between 1980 and 2000 are compared for different latitudes. The largest decreases have occurred at the highest latitudes in both hemispheres because of the large winter/spring depletion in polar regions. The losses in the Southern Hemisphere are greater than those in the Northern Hemisphere because of the greater losses that occur each year in the Antarctic stratosphere. Long-term changes in the tropics are much smaller because reactive halogen gases are not abundant in the tropical lower stratosphere.
As a result of the discovery of ozone depletion and the scientific advances that delineated its causes, efforts to reduce the production, and ultimately the atmospheric concentrations, of ozone-depleting chemicals were begun in the late 1980s through the ratification of the Montreal Protocol on Substances that Deplete the Ozone Layer by many countries across the globe. This international Protocol and its subsequent revisions and amendments have resulted in a turnaround in the atmospheric abundance of most ozone-depleting chemicals. While atmospheric levels of ozone-depleting chemicals were rapidly increasing before the Protocol was ratified, emissions of nearly all of these chemicals have declined substantially and atmospheric levels of most of these gases have decreased in the intervening 2 decades.
Measurements show that depletion of the ozone layer steadily worsened during the 1980s and most of the 1990s, but more recently as atmospheric amounts of chlorine and bromine have stabilized, a further worsening of ozone depletion appears to have been avoided. In the mid-latitude stratosphere, for example, the decreases in the ozone layer seen in the 1980s and 1990s have not continued.
Continued declines in ozone-depleting gases are expected to allow for a recovery of the ozone layer, but not until the middle of the 21st century. The long time scale for this recovery arises because ozone depleting gases such as chlorofluorocarbons are only removed from the atmosphere by natural processes at very slow rates.

Halogen source gas changes. The rise in effective stratospheric chlorine values in the 20th century has slowed and reversed in the last decade (top panel). Effective chlorine values combine the measured or projected abundances of chlorine-containing gases with those of brominecontaining gases in a way that properly accounts for the greater effectiveness of bromine in depleting stratospheric ozone. As effective chlorine decreases in the 21st century, the potential for ozone depletion from halogen gases will also decrease. The decrease in effective chlorine values is a result of reductions in individual halogen source gas emissions. The emissions decreased because of the Montreal Protocol, which restricts production and consumption of manufactured halogen gases. The changes in the atmospheric abundance of individual gases are shown in the lower panels using a combination of direct atmospheric measurements, estimates of historical abundance, and future projections of abundance. The increases of CFCs, along with those of CCl4 and CH3CCl3, have either slowed significantly or reversed in the last decade. HCFCs, which are being used as CFC substitutes, will continue to increase in the coming decades. Some halon abundances will also grow in the future while current halon reserves are being depleted. Smaller relative decreases are expected for CH3Br in response to restrictions because it has substantial natural sources. CH3Cl has large natural sources and is not regulated under the Montreal Protocol.
What don't we know?
Progress continues on reducing atmospheric amounts of chlorine and bromine so as to allow for the recovery of the ozone layer. Continued declines in emissions of chlorine and bromine gases are necessary for a full recovery of the ozone layer- but will occur only with strict adherence to the restrictions outlined in the fully revised and amended Protocol. Furthermore, future levels of bromine and chlorine depend upon natural processes removing these gases from the atmosphere as they have in the past, despite changes in atmospheric temperatures, circulation, etc. Hence, there exists uncertainty in how the levels of bromine and chlorine will change in the future.
If by 2050 bromine and chlorine returned to the levels present in 1980 and every other aspect of the atmospheric environment were unchanged, we would expect a full recovery of the ozone layer. Other aspects (temperature, winds, etc.) of the atmospheric environment and chemicals other than halocarbons can also influence the ozone layer. Changes in these features have been observed and will undoubtedly continue to change in the future. Because the interactions between ozone, temperature, mixing rates, water vapor (and other chemicals) are complex and multi-faceted, accurate predictions of the future ozone layer's health in the face of predicted or unexpected changes are difficult. Changes in ozone depletion may also affect climate change, and many of the chemicals involved in ozone depletion and their substitutes also can influence climate. These interactions at this time are very uncertain. Efforts are currently underway to explore the range of potential future atmospheric conditions and how they might influence the health of the ozone layer.
What is NOAA's role?
NOAA is responsible for monitoring the stratospheric ozone layer and ozone-depleting gases and it plays a large role in making the fundamental measurements of other atmospheric variables (water vapor, nitrous oxide, aerosols, etc.) that influence the ozone layer. NOAA scientists are leading efforts in assessing alternative chemicals for possible use as replacements to ozone depleting gases through laboratory study. NOAA is also responsible for synthesizing this information to allow for a comprehensive picture of the ozone layer, its changes, and how it might change in the future.
As a result of these activities, NOAA has led the effort to guide the recovery of the ozone layer, to ensure the recovery proceeds as expected, and to note areas or action that might allow a faster recovery or hinder recovery. Measurements of ozone-depleting gases provide a means by which adherence to international protocols can be assessed. Measurements of ozone allow one to discern if the policy actions are having their desired effect. Studies of potential climate change effects (e.g., changes in temperature, circulation, or the abundance of other chemicals) allow for much less ambiguity in accurately attributing any observed changes in the ozone layer to their appropriate cause.
These NOAA activities constitute a large part of the global scientific effort to understand stratospheric ozone depletion and recovery. NOAA's scientists not only are involved in maintaining a large portion of the world air sampling and measurement network, but also provide the calibration necessary for an integrated network and serve on several advisory groups and expert committees for assuring quality control, improving understanding, and identifying future needs. Only through careful management and interpretation of accurate, high-resolution measurements can we manage our environmental resources efficiently and effectively.
What will we need to know in the future?
Ozone depletion is still at its peak-ozone levels in many regions of the global are as low as they have ever been. Indications of a recovery are beginning to be seen, though we've a long way to go before the problem can be regarded as solved.
- Continued monitoring and process studies of ozone and ozone-depleting gases are required if we hope to be able to discern if the ozone layer is recovering as expected, or whether additional actions are necessary to ensure the recovery of the ozone layer.
- Continued scientific advances in understanding processes and their simulation in atmospheric models are needed to understand how secondary influences will affect ozone. This is particularly important as the burdens of Cl and Br diminish. These include the influences of a changing climate, altered air mixing and transport rates, energy exchange, and changes in the composition of the atmosphere (e.g., water vapor, methane, nitrous oxide, aerosols, etc.), all of which can influence stratospheric ozone.
- Only through additional study and incorporation in improved models will we accurately predict how the interplay of the multitude of factors affecting stratospheric ozone layer will respond to declines in chlorine and bromine.
What are the benefits for society of NOAA's activities?
NOAA's activities are focused on ensuring a recovery of the ozone layer so that it once again provides protection to all life from the Sun's harmful UV radiation. These activities can guarantee that the efforts heal the ozone layer stay on course and do not become sidetracked by unknown and unforeseen events or occurrences. Through its meticulous monitoring of the atmospheric composition and scientific expertise in understanding processes and modeling, as well as the search for CFC alternatives, NOAA provides much of the global scientific foundation for understanding the ozone layer and its changes.
Theme Presentations
- Ozone Introduction
- Laboratory Measurements
- Ozone-Depleting Substances
- Ozone Measurements
- Ozone/Climate Interactions - part 1, part 2
