Fulfilling Our Mission Through Development
Collaborations and New Technologies

Collaborations
- CCR Lab/Branch
- Trans-NCI
- Trans-NIH
- Intramural/Extramural
- Other Federal Agencies
- National and International Consortia
- Industry
- Pharmaceutical Companies
CCR recognizes the importance of building strong scientific partnerships. Our teams leverage their diverse strengths in various disciplines, approaches, technologies, and knowledge of specific diseases. By partnering with public and private institutions, we accelerate the speed at which we can bring scientific discoveries to the market place for the ultimate benefit of public health.
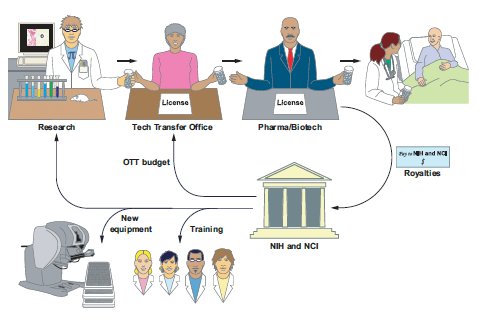
Reinvestment of Technology Development
Funds tech transfer, training, equipment, and research
CCR establishes CRADAs, patents, and licenses and reinvests the royalties it receives from its inventions to support training, new equipment purchases, and high-impact research.
Commercial Successes in Fighting Cancer and HIV
Gardasil®: A Vaccine against Human Papilloma Virus (HPV)
- Cervical cancer is linked to HPV infection.
- Clinical trials of Gardasil ® demonstrated 100 percent protection against the development of precancerous cervical lesions and nearly complete protection against the development of genital warts.
Kepivance ®: Improving the Quality of Life for Cancer Patients
- Mucositis (painful sores and ulcers in the lining of the mouth) affects ~80 percent of patients who undergo chemotherapy and/or radiation treatment prior to bone marrow transplantation, making eating, drinking, swallowing, and talking difficult or impossible.
- Prior to Kepivance ®, there was no treatment for this condition.
- Kepivance ® benefits ~11,000 adult Americans with hematologic malignancies who undergo bone marrow transplantation each year.
Here are some examples of drugs developed for patients with HIV/AIDS:
- Videx/Hivid ®: A Reverse Transcriptase Inhibitor
Treatments that selectively inhibit the replication of HIV by interfering with reverse transcriptase. - Prezista ®: A Protease Inhibitor
A small molecule HIV protease inhibitor used in combination with other antiretroviral agents and in antiretroviral treatment-experienced adult patients, such as those with HIV-1 strains resistant to more than one protease inhibitor. - Vitravene ®: An Antisense Drug
World’s first drug developed using antisense technology that offers an alternative for treatment of cytomegalovirus retinitis (CMV-R) in AIDS patients, and delays disease progression.
Vaccines and Therapeutics
2-F-AraA – Fludara (1991) Berlex
Videx® (1991) Berlex
Hivid® (1992) BMS
Paclitaxel® (1992) BMS
Trimetrexate – Neu Trexin (1993) US Bioscience
Zenapax® (1997) Hoffman La Roche
Vitravene® (1998) Isis Pharma
Zevalin® (2002) IDEC Pharma
Kepivance® (2004) Amgen
Gardasil® (2006) Merck
Prezista® (2006) Tibotec Pharma
Diagnostics
Serological Detection of Antibodies to HIV-1 (1985)
Serologic Detection of Antibodies to HTLV-1 (1988)
DNA Probe for Breast Cancer Diagnosis (1998)
Multi-Replica Blotting Kit for Proteins
Instrumentation/Devices
Laser Capture Microdissection
Research Tools Are Shared

Research Tools: The CCR contributed to the extramural research community by sharing nearly 1,000 research materials through material transfer agreements in 2007.
Shared research materials include cell lines, reagents, vaccines, antibodies, plasmids, clones, transgenic animals, assays, and software.

Excellence in Technology Transfer
When CCR scientists produce research tools, technology, and inventions, in-house legal staff help them file invention reports, license their technology, and establish cooperative research or materials transfer agreements. This enables innovative intramural researchers to share their intellectual property with the extramural community.




